formylpiperazine
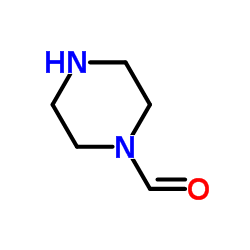
formylpiperazine structure
|
Common Name | formylpiperazine | ||
|---|---|---|---|---|
| CAS Number | 7755-92-2 | Molecular Weight | 114.146 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 291.7±0.0 °C at 760 mmHg | |
| Molecular Formula | C5H10N2O | Melting Point | 88-91 | |
| MSDS | Chinese USA | Flash Point | 101.7±0.0 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Synthesis and receptor binding assay of indolin-2-one derivatives as dopamine D4 receptor ligands.
Pharmazie 70 , 511-4, (2015) Five indolin-2-one derivatives bearing piperazinylbutyl side chains attached to the amide nitrogen were synthesized from 2-indolinone. 1-(4-Bromobutyl)-indolin-2-one was reacted with 1-piperazinecarboxaldehyde to form 1-(4-(4-formyl-1-piperazinyl)butyl)indoli... |
|
|
Infrared and Raman spectra, conformational stability and vibrational assignment of 1-formylpiperazine.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 118 , 1113-1120, (2014) Infrared and Raman spectra of 1-formylpiperazine (1-fp) have been recorded in the region of 4000-50 cm(-1). The conformational analysis, optimized geometric parameters, normal mode frequencies and corresponding vibrational assignments of 1-fp (C5H10N2O) have ... |
|
|
1-Formylpiperazine and related compounds. Horrom BW, et al.
J. Am. Chem. Soc. 77(3) , 753-754, (1955)
|
|
|
Reactions of O-aryl S-aryl dithiocarbonates with secondary alicyclic amines in aqueous ethanol. Kinetics and mechanism. Castro EA, et al.
J. Phys. Org. Chem. 24(6) , 466-473, (2011)
|
|
|
Kinetics and mechanism for the reaction of 4-nitrophenyl 2-thiophenecarboxylate with secondary alicyclic amines. Um IH, et al.
Bull. Korean Chem. Soc. 23(3) , 381-384, (2002)
|
|
|
Kinetics and mechanism of the aminolysis of O-ethyl S-aryl dithiocarbonates. Oh HK, et al.
J. Org. Chem. 56(18) , 5324-5328, (1991)
|
|
|
Thiocarbamoyl derivatives of N-acylpiperazines. Dhawan B and Southwick PL.
J. Heterocycl. Chem. 20(1) , 243-244, (1983)
|
|
|
Influence of the ionic liquid on the rate and the mechanism of reaction of p-nitrophenyl acetate with secondary alicyclic amines. Millán D, et al.
New J. Chem. 37(10) , 3281-3288, (2013)
|