Furaltadone
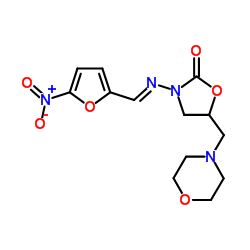
Furaltadone structure
|
Common Name | Furaltadone | ||
|---|---|---|---|---|
| CAS Number | 139-91-3 | Molecular Weight | 324.289 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 461.4±55.0 °C at 760 mmHg | |
| Molecular Formula | C13H16N4O6 | Melting Point | 206ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 232.8±31.5 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Elucidating the regulon of multidrug resistance regulator RarA in Klebsiella pneumoniae.
Antimicrob. Agents Chemother. 57(4) , 1603-9, (2013) RarA is an AraC-type regulator in Klebsiella pneumoniae, which, when overexpressed, confers a low-level multidrug-resistant (MDR) phenotype linked to the upregulation of both the acrAB and oqxAB efflux genes. Increased rarA expression has also been shown to b... |
|
|
Development of a competitive ELISA for the detection of a furaltadone marker residue, 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ), in cultured fish samples.
J. Vet. Med. Sci. 74(11) , 1439-46, (2012) This report describes an enzyme-linked immunosorbent assay (ELISA) for tissue-bound metabolite 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ) and the application to residue analysis in cultured fish samples. The residue is monitored as a marker for the dru... |
|
|
Cloning, expression, purification, and characterization of a novel single-chain variable fragment antibody against the 2-nitrobenzaldehyde derivative of a furaltadone metabolite in Escherichia coli.
Protein Expr. Purif. 84(1) , 140-6, (2012) Furaltadone is an illicit veterinary drug that shows toxic, carcinogenic, and mutagenic effects, as does its metabolite 3-amino-5-morpholinomethyl-2-oxazolidone (AMOZ)(1). Recombinant antibodies with desirable affinity and specificity that can replace polyclo... |
|
|
AAS and AES determination of furaltadone, methadone and trazodone in pharmaceutical formulations.
J. Pharm. Biomed. Anal. 27(1-2) , 117-22, (2002) Ion-associate complexes of furaltadone, methadone and trazodone hydrochlorides with [Cd(SCN)(4)](2-) and [Zn(SCN)(4)](2-) were precipitated and the excess unreacted cadmium or zinc complex was determined. A new method using atomic emission and atomic absorpti... |
|
|
Allergic contact dermatitis from furaltadone in eardrops.
Contact Dermatitis 40(4) , 222, (1999)
|
|
|
Determination of nitrofurantoin, furazolidone and furaltadone in milk by high-performance liquid chromatography with electrochemical detection.
J. Chromatogr. A. 764(2) , 243-8, (1997) A HPLC method with coulometric detection has been established to carry out the separation of the three nitrofuran derivatives, nitrofurantoin, furazolidone and furaltadone. A Nova-Pak C18 column (150 x 3.9 mm) and a Coulochem II detector from ESA have been us... |
|
|
Differential effects of nitrofurans on the production/release of steroid hormones by porcine adrenocortical cells in vitro.
Eur. J. Pharmacol. 331(2-3) , 325-31, (1997) Changes in the biogenesis of corticosteroids caused by nitrofurans were studied. The three nitrofurans used: furazolidone, furaltadone and nitrofurantoin, altered the steroid production/release by porcine adrenocortical cells in vitro during 1 h incubations. ... |
|
|
Resolution of ternary mixtures of nitrofurantoin, furaltadone and furazolidone by partial least-square analysis to the spectrophotometric signals after photo-decomposition.
J. Pharm. Biomed. Anal. 29(3) , 477-85, (2002) An UV spectroscopic method is proposed to analyze mixtures of the nitrofuran derivatives, nitrofurantoin, furaltadone and furazolidone, used in veterinary. The change of absorption spectra due to photo-decomposition is used. A 20% dimethylformamide/water, bas... |
|
|
A LC-MS/MS methodology to determine furaltadone residues in the macroalgae Ulva lactuca.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879(32) , 3832-6, (2011) Presently, the rise of new contaminants in the environment has widened the scope of pharmaceutical analyses as to face the demanding new challenges. An increasing tendency for the interconnection and overlap of research fields, such as ecology and biochemistr... |
|
|
Development of an enzyme-linked immunosorbent assay for determination of the furaltadone etabolite, 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ) in animal tissues.
Biomed. Environ. Sci. 25(4) , 449-57, (2012) To determine 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ) residues released from protein bound AMOZ in animal tissues.Polyclonal and monoclonal antibodies were produced in this study. A rapid, sensitive, and specific competitive direct enzyme-linked immu... |