1-(2-Bromoacetoxy)-2,5-pyrrolidinedione
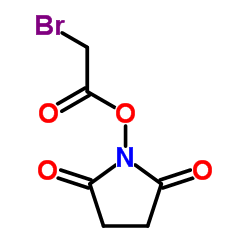
1-(2-Bromoacetoxy)-2,5-pyrrolidinedione structure
|
Common Name | 1-(2-Bromoacetoxy)-2,5-pyrrolidinedione | ||
|---|---|---|---|---|
| CAS Number | 42014-51-7 | Molecular Weight | 236.020 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 291.7±42.0 °C at 760 mmHg | |
| Molecular Formula | C6H6BrNO4 | Melting Point | 115-116ºC | |
| MSDS | USA | Flash Point | 130.2±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Preparation of peptide-protein immunogens using N-succinimidyl bromoacetate as a heterobifunctional crosslinking reagent.
Anal. Biochem. 155 , 95-102, (1986) Synthetic peptides derived from human fibrin were unidirectionally conjugated to three carrier proteins (bovine serum albumin, bovine alpha-lactalbumin, and keyhole limpet hemocyanin) by a method that employs N-succinimidyl bromoacetate. This heterobifunction... |
|
|
Production of antibodies against degradative neoepitopes in aggrecan.
Methods Mol. Med. 100 , 237-50, (2004) The use of synthetic peptides to generate rabbit polyclonal anticatabolic neoepitope antibodies that can be used to study the presence of defined proteolytic cleavage sites in aggrecan is described. Principles of peptide design and methods for preparation and... |
|
|
Conjugation of synthetic peptides to proteins: quantitation from S-carboxymethylcysteine released upon acid hydrolysis.
Anal. Biochem. 187(1) , 136-40, (1990) A method described here for conjugating synthetic peptides to carrier proteins provides a convenient method for determining peptide-to-carrier protein ratios. N-Bromoacetyl-containing peptides are reacted in situ with carrier proteins in which the disulfide b... |