Flunixin Meglumine
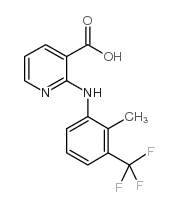
Flunixin Meglumine structure
|
Common Name | Flunixin Meglumine | ||
|---|---|---|---|---|
| CAS Number | 38677-85-9 | Molecular Weight | 296.24500 | |
| Density | 1.403g/cm3 | Boiling Point | 378.7ºC at 760 mmHg | |
| Molecular Formula | C14H11F3N2O2 | Melting Point | 226 - 228ºC | |
| MSDS | Chinese USA | Flash Point | 182.8ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Multiresidue method for the determination of pharmacologically active substances in egg and honey using a continuous solid-phase extraction system and gas chromatography-mass spectrometry.
Food Chem. 178 , 63-9, (2015) A sensitive, selective, efficient gas chromatography-mass spectrometry method for the simultaneous determination of 22 pharmacologically active substances (antibacterials, nonsteroidal antiinflammatories, antiseptics, antiepileptics, lipid regulators, β-block... |
|
|
Drug-induced mild therapeutic hypothermia obtained by administration of a transient receptor potential vanilloid type 1 agonist.
BMC Cardiovasc. Disord. 10 , 51, (2010) The use of mechanical/physical devices for applying mild therapeutic hypothermia is the only proven neuroprotective treatment for survivors of out of hospital cardiac arrest. However, this type of therapy is cumbersome and associated with several side-effects... |
|
|
Screening and Confirmatory Analyses of Flunixin in Tissues and Bodily Fluids after Intravenous or Intramuscular Administration to Cull Dairy Cows with or without Lipopolysaccharide Challenge.
J. Agric. Food Chem. 64 , 336-45, (2016) Twenty cull dairy cows (645 ± 83 kg) were treated with 2.2 mg/kg bw flunixin by intravenous (IV) or intramuscular (IM) administration with, or without, exposure to lipopolysaccharide in a two factor balanced design. The usefulness of screening assays to ident... |
|
|
Pig skin includes dendritic cell subsets transcriptomically related to human CD1a and CD14 dendritic cells presenting different migrating behaviors and T cell activation capacities.
J. Immunol. 193(12) , 5883-93, (2014) Swine skin is one of the best structural models for human skin, widely used to probe drug transcutaneous passage and to test new skin vaccination devices. However, little is known about its composition in immune cells, and among them dendritic cells (DC), tha... |
|
|
The effect of breed and sex on sulfamethazine, enrofloxacin, fenbendazole and flunixin meglumine pharmacokinetic parameters in swine.
J. Vet. Pharmacol. Ther. 37(6) , 531-41, (2014) Drug use in livestock has received increased attention due to welfare concerns and food safety. Characterizing heterogeneity in the way swine populations respond to drugs could allow for group-specific dose or drug recommendations. Our objective was to determ... |
|
|
A comparative study on the adverse effects of flunixin, ketoprofen and phenylbutazone in miniature donkeys: haematological, biochemical and pathological findings.
N. Z. Vet. J. 58(5) , 224-8, (2010) To evaluate the adverse effects of flunixin, ketoprofen and phenylbutazone when administered I/V to clinically normal miniature donkeys.Twenty clinically normal adult (2.0-2.5 years old) male miniature donkeys weighing 113-136 kg and 0.81- 0.86 m tall were ra... |
|
|
Pharmacokinetics of flunixin in chickens after oral and intravenous administration.
J. Vet. Pharmacol. Ther. 33(3) , 312-4, (2010)
|
|
|
In-house reference materials: 5-hydroxyflunixin and meloxicam in cow milk—preparation and evaluation
Anal. Chim. Acta 637(1-2) , 346-50, (2009) Reference materials are helpful to evaluate the performance of laboratories as well as being useful for the quality control of analytical procedures. Certified reference materials and other reference materials containing non-steroidal anti-inflammatory drugs ... |
|
|
Efficacy of ceftiofur and flunixin in the early treatment of bronchopneumonia in weaners.
Vet. Rec. 158(9) , 291-6, (2006) Three groups of five pigs were inoculated intratracheally with Escherichia coli lipopolysaccharides, and 24 hours later with 10 x 10(9) colony-forming units of a non-toxigenic strain of Pasteurella multocida type A; a fourth group was left uninoculated as con... |
|
|
Effect of day of transfer and treatment administration on the recipient on pregnancy rates after equine embryo transfer.
Vet. Res. Commun. 33 Suppl 1 , 113-6, (2009)
|