1,3-Dithiane
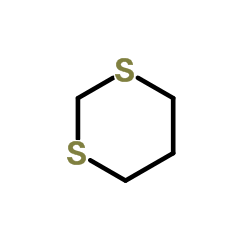
1,3-Dithiane structure
|
Common Name | 1,3-Dithiane | ||
|---|---|---|---|---|
| CAS Number | 505-23-7 | Molecular Weight | 120.236 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 195.0±23.0 °C at 760 mmHg | |
| Molecular Formula | C4H8S2 | Melting Point | 52-54 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 90.6±0.0 °C | |
|
Genotoxicity of 1,3-dithiane and 1,4-dithiane in the CHO/SCE assay and the Salmonella/microsomal test.
Mutat. Res. 321(4) , 213-8, (1994) 1,3-Dithiane and 1,4-dithiane are the sulfur-containing Maillard reaction products (MRPs) which have been found in boiled beef extracts. In this study the genotoxicity of these products was examined using the Salmonella/microsomal test and the CHO/SCE assay. ... |
|
|
Synthesis and applications of masked oxo-sulfinamides in asymmetric synthesis.
Org. Biomol. Chem. 10(26) , 5021-31, (2012) This short perspective reports on the synthesis and applications of a class of chiral amino carbonyl compounds, masked oxo-sulfinamides where the amine is protected with an N-sulfinyl moiety and the carbonyl group is protected as the ketal or 1,3-dithiane. Th... |
|
|
Au(PPh(3))Cl-AgSbF(6)-catalyzed rearrangement of propargylic 1,3-dithianes: formation of 8-membered 1,3-bisthio-substituted cyclic allenes.
Chem. Commun. (Camb.) (18) , 2535-7, (2009) Au(PPh(3))Cl-AgSbF(6)-catalyzed rearrangement of propargylic 1,3-dithiane leads to the formation of 8-membered dithio-substituted cyclic allenes, which are remarkably stable. |
|
|
A dimethyl ketal-protected benzoin-based linker suitable for photolytic release of unprotected peptides.
J. Org. Chem. 76(22) , 9409-16, (2011) Photolabile 3',5'-dimethoxybenzoin-based linkers are advantageous for a variety of solid-phase synthetic procedures and manipulations of biomolecules because UV irradiation in aqueous media provides fast and essentially quantitative release of tethered molecu... |
|
|
A new approach to the reduction of sulfoxides to sulfides with 1,3-dithiane in the presence of electrophilic bromine as catalyst.
J. Org. Chem. 67(9) , 2826-30, (2002) A new, mild, and novel method is described for the efficient deoxygenation of sulfoxides to their corresponding sulfides with 1,3-dithiane at room temperature in the presence of catalytic amounts of N-bromosuccinimide (NBS), 2,4,4,6-tetrabromo-2,5-cyclohexadi... |
|
|
Synthesis of isotopically labeled 1,3-dithiane.
J. Labelled Comp. Radiopharm. 57(5) , 338-41, (2014) The 1,3-dithiane is a protected formaldehyde anion equivalent that could serve as a useful labeled synthon. We report a facile synthesis of 1,3-[2-(13)C]- and 1,3-[2-(13)C, 2-(2)H2]dithiane in two steps from [(13)C]- or [(13) C, (2)H3 ]methyl phenyl sulfoxide... |
|
|
Lewis base catalyzed 1,3-dithiane addition to carbonyl and imino compounds using 2-trimethylsilyl-1,3-dithiane.
Chem. Asian J. 3(8-9) , 1592-600, (2008) Lewis base-catalyzed 1,3-dithiane addition to electrophiles such as carbonyl compounds and N-substituted aldimines with 2-trimethylsilyl-1,3-dithiane (TMS-dithiane) is described. By the activation of the carbon-silicon bond in the presence of a Lewis base cat... |
|
|
A new "turn-on" chemodosimeter for Hg2+: ICT fluorophore formation via Hg(2+)-induced carbaldehyde recovery from 1,3-dithiane.
Chem. Commun. (Camb.) 48(42) , 5094-6, (2012) A novel sensitive and specific Hg(2+) chemodosimeter, derived from 1',3'-dithiane-substituted 2,1,3-benzoxadiazole, displays "turn-on" fluorescent and colorimetric responses via an Hg(2+)-triggered aldehyde recovery reaction. Its potential to monitor Hg(2+) i... |
|
|
Asymmetric synthesis of α-amino-1,3-dithianes via chiral N-phosphonyl imine-based Umpolung reaction without using chromatography and recrystallization.
J. Org. Chem. 76(8) , 2792-7, (2011) A series of α-amino-1,3-dithianes have been synthesized via the asymmetric Umpolung reaction of 2-lithio-1,3-dithianes with chiral N-phosphonyl imines in good chemical yields (up to 82%) and good to excellent diastereoselectivities (>99:1). The manner by whic... |
|
|
Use of 1,3-dithiane combined with aryldiazonium cation for immobilization of biomolecules based on electrochemical addressing.
Chem. Commun. (Camb.) (32) , 4865-7, (2009) We report the use of 1,3-dithiane combined with aryldiazonium cation for the immobilization of biomolecules based on electrochemical addressing. |