Asymmetric synthesis of α-amino-1,3-dithianes via chiral N-phosphonyl imine-based Umpolung reaction without using chromatography and recrystallization.
Padmanabha V Kattamuri, Teng Ai, Suresh Pindi, Yinwei Sun, Peng Gu, Min Shi, Guigen Li
Index: J. Org. Chem. 76(8) , 2792-7, (2011)
Full Text: HTML
Abstract
A series of α-amino-1,3-dithianes have been synthesized via the asymmetric Umpolung reaction of 2-lithio-1,3-dithianes with chiral N-phosphonyl imines in good chemical yields (up to 82%) and good to excellent diastereoselectivities (>99:1). The manner by which chiral N-phosphonyl imines are slowly added into the solution of 2-lithio-1,3-dithiane was found to be crucial for achieving excellent diastereoselectivity. The current synthesis was proven to follow the GAP chemistry (group-assistant-purification chemistry) process, which avoids traditional purification techniques of chromatography or recrystallization, i.e., the pure chiral α-amino-1,3-dithianes attached with the chiral N-phosphonyl group were readily obtained by washing the solid crude products with hexane or a mixture of hexane-ethyl acetate.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
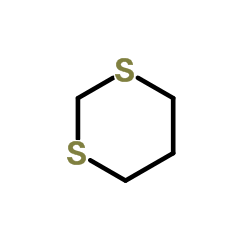 |
1,3-Dithiane
CAS:505-23-7 |
C4H8S2 |
|
Genotoxicity of 1,3-dithiane and 1,4-dithiane in the CHO/SCE...
1994-06-01 [Mutat. Res. 321(4) , 213-8, (1994)] |
|
Synthesis and applications of masked oxo-sulfinamides in asy...
2012-07-14 [Org. Biomol. Chem. 10(26) , 5021-31, (2012)] |
|
Au(PPh(3))Cl-AgSbF(6)-catalyzed rearrangement of propargylic...
2009-05-14 [Chem. Commun. (Camb.) (18) , 2535-7, (2009)] |
|
A dimethyl ketal-protected benzoin-based linker suitable for...
2011-11-18 [J. Org. Chem. 76(22) , 9409-16, (2011)] |
|
A new approach to the reduction of sulfoxides to sulfides wi...
2002-05-03 [J. Org. Chem. 67(9) , 2826-30, (2002)] |