alpha-Terthiophene
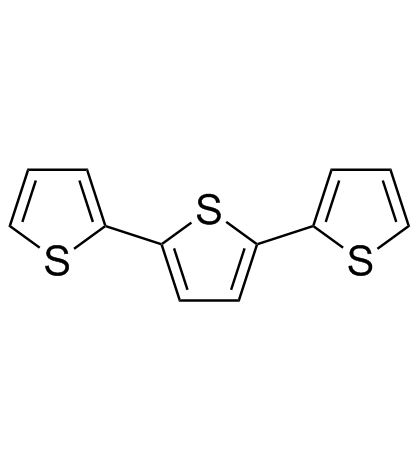
alpha-Terthiophene structure
|
Common Name | alpha-Terthiophene | ||
|---|---|---|---|---|
| CAS Number | 1081-34-1 | Molecular Weight | 248.387 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 361.3±32.0 °C at 760 mmHg | |
| Molecular Formula | C12H8S3 | Melting Point | 93-95 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 128.4±11.3 °C | |
|
Length-dependent conductance of oligothiophenes.
J. Am. Chem. Soc. 136(29) , 10486-92, (2014) We have measured the single-molecule conductance of a family of oligothiophenes comprising 1-6 thiophene moieties terminated with methyl-sulfide linkers using the scanning tunneling microscope-based break-junction technique. We find an anomalous behavior: the... |
|
|
Radical-scavenging activity of melatonin, either alone or in combination with vitamin E, ascorbate or 2-mercaptoethanol as co-antioxidants, using the induction period method.
In Vivo 25(1) , 49-53, (2011) Melatonin shows antioxidant/prooxidant activity but its mechanism of action remains unknown.The radical-scavenging activity of melatonin and various melatonin/co-antioxidant mixtures in a 1:1 molar ratio was evaluated in terms of the length of the induction p... |
|
|
Synthesis and evaluation of new spacers for use as dsDNA end-caps.
Bioconjug. Chem. 21(8) , 1545-53, (2010) A series of aliphatic and aromatic spacer molecules designed to cap the ends of DNA duplexes have been synthesized. The spacers were converted into dimethoxytrityl-protected phosphoramidites as synthons for oligonucleotides synthesis. The effect of the spacer... |
|
|
p-i-n Homojunction in organic light-emitting transistors.
Adv. Mater. 23(24) , 2753-8, (2011)
|
|
|
The synthesis and photoactivated cytotoxicity of 2-methyl-4-oxo-3-prop-2-yn-1-ylcyclopent-2-en-1-yl-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate conjugated with alpha-terthienyl derivatives.
J. Photochem. Photobiol. B, Biol. 96(3) , 170-7, (2009) The synthesis of one pyrethroid insecticide [2-methyl-4-oxo-3-prop-2-yn-1-ylcyclopent-2-en-1-yl-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (Abbrev. JZ) (Fig. 1)] conjugated with a series of alpha-terthienyl derivatives (2-8) (Fig. 1) by palladi... |
|
|
Spectroscopy and single-molecule emission of a fluorene-terthiophene oligomer.
J. Phys. Chem. B 115(42) , 12028-35, (2011) We study the thiophene-based oligomer poly[2,7-(9,9-bis(2'-ethylhexyl)fluorene)-alt-2,5-terthiophene] (PF3T) in solution and when dispersed at low concentration into a polynorbornene matrix. We find that at high concentration in solution the 0-0 electronic tr... |
|
|
Chemical fingerprint of commercial Radix Echinopsis and quantitative analysis of alpha-terthienyl.
J. Sep. Sci. 33(4-5) , 530-8, (2010) New TLC, HPLC and LC/MS methods were developed for the rapid separation, characterization and quantitation of thiophenes in Radix Echinopsis, a herbal medicine, which has been used in China for long history. Nineteen commercial batches of this herb were analy... |
|
|
Triplet excited state characters and photosensitization mechanisms of α-terthienyl: A theoretical study
J. Photochem. Photobiol. B, Biol. 94(1) , 51-3, (2009) The triplet excited (T 1) state characters of α-terthienyl (α-T) have been investigated using density functional theory calculations, based on which, its photosensitization mechanisms were explored. Primarily, the direct oxidation to the DNA bases by the T 1 ... |
|
|
Solid-state synthesis of poly(3',4'-dimethoxy-2,2':5',2"- terthiophene): comparison with poly(terthiophene) and poly(3',4'-ethylenedioxy-2,2':5',2"- terthiophene).
Molecules 17(7) , 8647-60, (2012) A new terthiophene monomer: 3',4'-dimethoxy-2,2':5',2"-terthiophene (TMT) was synthesized and characterized by ¹H-NMR, ¹³C-NMR and FTIR. The solid-state oxidative polymerizations of TMT were performed in various ratios of oxidant (FeCl₃) to monomer (TMT). The... |
|
|
Excess-electron injection and transfer in terthiophene-modified DNA: terthiophene as a photosensitizing electron donor for thymine, cytosine, and adenine.
Chemistry 18(7) , 2056-62, (2012) Excess-electron transfer (EET) in DNA has attracted wide attention owing to its close relation to DNA repair and nanowires. To clarify the dynamics of EET in DNA, a photosensitizing electron donor that can donate an excess electron to a variety of DNA sequenc... |