Spectroscopy and single-molecule emission of a fluorene-terthiophene oligomer.
G E Khalil, A M Adawi, B Robinson, A J Cadby, W C Tsoi, J-S Kim, A Charas, J Morgado, D G Lidzey
Index: J. Phys. Chem. B 115(42) , 12028-35, (2011)
Full Text: HTML
Abstract
We study the thiophene-based oligomer poly[2,7-(9,9-bis(2'-ethylhexyl)fluorene)-alt-2,5-terthiophene] (PF3T) in solution and when dispersed at low concentration into a polynorbornene matrix. We find that at high concentration in solution the 0-0 electronic transition observed in fluorescence is suppressed, a result indicative of the formation of weakly coupled H-aggregates. At low concentration in a polymer matrix, emission from both single molecules and molecular aggregates is observed. We find that the fluorescence spectra of most PF3T emitters are composed of a number of relatively narrow emission features, indicating that the emission usually occurs from multiple chromophores. A small number of PF3T molecules are however characterized by single chromophore emission, spectral blinking, and narrowed emission peaks.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
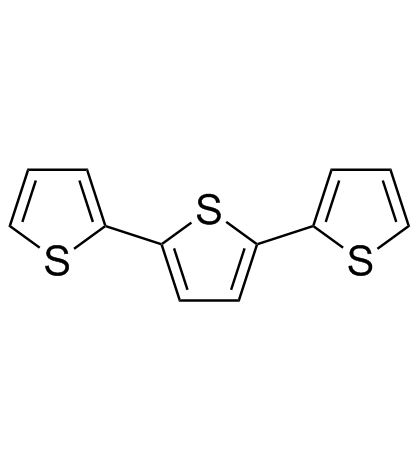 |
alpha-Terthiophene
CAS:1081-34-1 |
C12H8S3 |
|
Length-dependent conductance of oligothiophenes.
2014-07-23 [J. Am. Chem. Soc. 136(29) , 10486-92, (2014)] |
|
Radical-scavenging activity of melatonin, either alone or in...
2011-01-01 [In Vivo 25(1) , 49-53, (2011)] |
|
Synthesis and evaluation of new spacers for use as dsDNA end...
2010-08-18 [Bioconjug. Chem. 21(8) , 1545-53, (2010)] |
|
p-i-n Homojunction in organic light-emitting transistors.
2011-06-24 [Adv. Mater. 23(24) , 2753-8, (2011)] |
|
The synthesis and photoactivated cytotoxicity of 2-methyl-4-...
2009-09-04 [J. Photochem. Photobiol. B, Biol. 96(3) , 170-7, (2009)] |