4,5,6,7-Tetrachloro-2-hydroxy-isoindole-1,3-dione
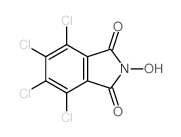
4,5,6,7-Tetrachloro-2-hydroxy-isoindole-1,3-dione structure
|
Common Name | 4,5,6,7-Tetrachloro-2-hydroxy-isoindole-1,3-dione | ||
|---|---|---|---|---|
| CAS Number | 85342-65-0 | Molecular Weight | 300.91000 | |
| Density | 2.041g/cm3 | Boiling Point | 532.1ºC at 760mmHg | |
| Molecular Formula | C8HCl4NO3 | Melting Point | N/A | |
| MSDS | Chinese | Flash Point | 275.6ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
|
Efficient metal-free aerobic oxidation of aromatic hydrocarbons utilizing aryl-tetrahalogenated N-hydroxyphthalimides and 1,4-diamino-2,3-dichloroanthraquinone. Zhang Q, et al.
J. Chem. Technol. Biotechnol. 83(10) , 1364-1369, (2008)
|
|
|
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids.
Angew. Chem. Int. Ed. Engl. 55(33) , 9676-9, (2016) A transformation analogous in simplicity and functional group tolerance to the venerable Suzuki cross-coupling between alkyl-carboxylic acids and boronic acids is described. This Ni-catalyzed reaction relies upon the activation of alkyl carboxylic acids as th... |
|
|
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents.
Science 352(6287) , 801-5, (2016) Alkyl carboxylic acids are ubiquitous in all facets of chemical science, from natural products to polymers, and represent an ideal starting material with which to forge new connections. This study demonstrates how the same activating principles used for decad... |
|
|
Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters.
.PubMed ID A new transformation is presented that enables chemists to couple simple alkyl carboxylic acids with aryl zinc reagents under Ni-catalysis. The success of this reaction hinges on the unique use of redox-active esters that allow one to employ such derivatives ... |