dimethyl pimelimidate dihydrochloride
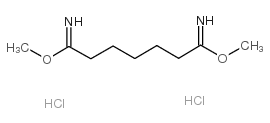
dimethyl pimelimidate dihydrochloride structure
|
Common Name | dimethyl pimelimidate dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 58537-94-3 | Molecular Weight | 259.17300 | |
| Density | N/A | Boiling Point | 301.9ºC at 760mmHg | |
| Molecular Formula | C9H20Cl2N2O2 | Melting Point | ~122 °C (dec.) | |
| MSDS | Chinese USA | Flash Point | 136.4ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Xenopus HJURP and condensin II are required for CENP-A assembly.
J. Cell Biol. 192(4) , 569-82, (2011) Centromeric protein A (CENP-A) is the epigenetic mark of centromeres. CENP-A replenishment is necessary in each cell cycle to compensate for the dilution associated to DNA replication, but how this is achieved mechanistically is largely unknown. We have devel... |
|
|
Cross-linking of the components of lactose synthetase with dimethylpimelimidate.
J. Biol. Chem. 250 , 1434-1444, (1975) The cross-linking of the two components of lactose synthetase, alpha-lactalbumin and a galactosyltransferase, with dimethylpimelimidate was examined. The extent of the cross-linking at pH 8.1 was found to be dependent upon the presence of substrates or inhibi... |
|
|
Analysis and isolation of human transferrin receptor using the OKT-9 monoclonal antibody covalently crosslinked to magnetic beads.
Anal. Biochem. 199 , 219-222, (1991) A method is described for the use of magnetic beads as a solid phase for the immunoprecipitation of labeled proteins. The anti-human transferrin receptor monoclonal antibody OKT-9 has been coupled to sheep anti-mouse IgG1-coated magnetic beads using the cross... |
|
|
One-step purification of Enterocytozoon bieneusi spores from human stools by immunoaffinity expanded-bed adsorption.
J. Clin. Microbiol. 39 , 1947-1951, (2001) An original, reliable, and reproducible method for the purification of Enterocytozoon bieneusi spores from human stools is described. We recently reported the production of a species-specific monoclonal antibody (MAb) 6E52D9 immunoglobulin G2a (IgG2a) raised ... |
|
|
Semisynthetic fluorohydrolases prepared by chemical modification of ribonuclease. David, E., et al.
Enz. Microbiol. Technol. 14 , 885-892, (1992)
|