Dutasteride
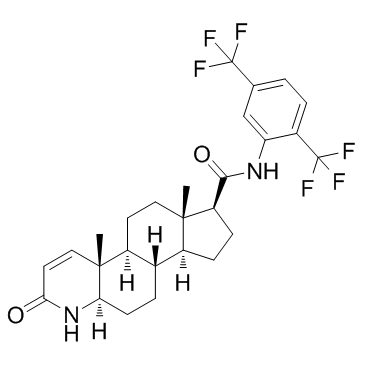
Dutasteride structure
|
Common Name | Dutasteride | ||
|---|---|---|---|---|
| CAS Number | 164656-23-9 | Molecular Weight | 528.530 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 620.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C27H30F6N2O2 | Melting Point | 242-250ºC | |
| MSDS | Chinese USA | Flash Point | 329.0±31.5 °C | |
|
Next-generation steroidogenesis inhibitors, dutasteride and abiraterone, attenuate but still do not eliminate androgen biosynthesis in 22RV1 cells in vitro
J. Steroid Biochem. Mol. Biol. 144 Pt B , 436-44, (2014) • Dutasteride and abiraterone were evaluated for inhibition of steroidogenesis. • Bypass mechanisms arise in the presence of abiraterone to form DHT. • Dutasteride inhibits T and DHT effectively in vitro. • Abiraterone inhibits AR and steroidogenesis leading ... |
|
|
Preparation of microcapsules with the evaluation of physicochemical properties and molecular interaction.
Arch. Pharm. Res. 37(12) , 1570-7, (2014) The objective of this study was to prepare and characterize dutasteride (a hydrophobic model drug) microcapsules using ethyl cellulose as a capsule shell polymer with different drug/polymer ratios of 1:1, 1:3, and 1:5. The microcapsules were prepared by a sol... |
|
|
Long term effectiveness on prescribing of two multifaceted educational interventions: results of two large scale randomized cluster trials.
PLoS ONE 9(10) , e109915, (2014) Information on benefits and risks of drugs is a key element affecting doctors' prescribing decisions. Outreach visits promoting independent information have proved moderately effective in changing prescribing behaviours.Testing the short and long-term effecti... |
|
|
Aspirin, NSAIDs, and risk of prostate cancer: results from the REDUCE study.
Clin. Cancer Res. 21(4) , 756-62, (2015) A recent meta-analysis showed that aspirin was associated with reduced prostate cancer risk. As anti-inflammatory medications lower PSA levels, whether these findings reflect reduced prostate cancer detection or lower prostate cancer risk is unknown. We teste... |
|
|
Under-representation of racial minorities in prostate cancer studies submitted to the US Food and Drug Administration to support potential marketing approval, 1993-2013.
Cancer 120(19) , 3025-32, (2014) US Food and Drug Administration (FDA) approval of new drugs depends on results from clinical trials that must be generalized to the US population. However, racial minorities are frequently under-represented in clinical studies. The enrollment of racial minori... |
|
|
Histologic evaluation of human benign prostatic hyperplasia treated by dutasteride: a study by xenograft model with improved severe combined immunodeficient mice.
Urology 85(1) , 274.e1-8, (2015) To evaluate histologic change in human prostate samples treated with dutasteride and to elucidate direct effects of dutasteride on human prostate tissue, the present study was conducted by using a xenograft model with improved severe combined immunodeficient ... |
|
|
Dutasteride for the treatment of benign prostatic hyperplasia.
Expert Opin. Pharmacother. 14(10) , 1399-408, (2013) Benign prostatic hyperplasia (BPH) is an age-related phenomenon associated with prostatic enlargement and bladder outlet obstruction that can cause significant lower urinary tract symptoms that greatly affect quality of life. Dutasteride is a selective inhibi... |
|
|
Impact of dutasteride on nocturia in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH): a pooled analysis of three phase III studies.
World J. Urol. 32(5) , 1141-7, (2014) To assess the impact of dutasteride compared with placebo on nocturia in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia, using pooled data from dutasteride phase III studies.Nocturia was assessed using Question 7 of the Inter... |
|
|
Nocturia improvement in the combination of Avodart(®) and tamsulosin (CombAT) study.
World J. Urol. 32(5) , 1133-40, (2014) The purpose of the study was to assess the impact of dutasteride plus tamsulosin combination therapy, compared with dutasteride or tamsulosin monotherapy, on nocturia in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BP... |
|
|
Effect of dutasteride in men receiving intermittent androgen ablation therapy: The AVIAS trial.
Can. Urol. Assoc. J. 8(11-12) , E789-94, (2014) We studied the effect of dutasteride on the length of the off-treatment period in prostate cancer patients on intermittent androgen deprivation (IAD) therapy.We conducted a randomized, placebo-controlled Phase II trial in men with localized prostate cancer an... |