2-Chloroacrylonitrile
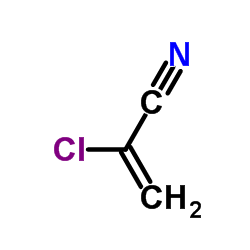
2-Chloroacrylonitrile structure
|
Common Name | 2-Chloroacrylonitrile | ||
|---|---|---|---|---|
| CAS Number | 920-37-6 | Molecular Weight | 87.508 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 89.5±13.0 °C at 760 mmHg | |
| Molecular Formula | C3H2ClN | Melting Point | −65 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 6.7±0.0 °C | |
| Symbol |





GHS02, GHS05, GHS06, GHS08, GHS09 |
Signal Word | Danger | |
|
Structure-nephrotoxicity relationships of glutathione pathway intermediates derived from organic solvents.
Toxicology 56(1) , 47-61, (1989) The nephrotoxicity of glutathione (GSH) pathway metabolites derived from toluene (TOL), styrene (STYR), bromobenzene (BB), acrylonitrile (ACLN) and 2-chloroacrylonitrile (CACLN) were compared with that of dichlorovinylcysteine (DCVC), using renal brush border... |
|
|
[Information from the Soviet Toxicology Center].
Gig. Tr. Prof. Zabol. (2) , 52-3, (1983)
|
|
|
Dual-function cinchona alkaloid catalysis: catalytic asymmetric tandem conjugate addition-protonation for the direct creation of nonadjacent stereocenters.
J. Am. Chem. Soc. 128(12) , 3928-30, (2006) Catalytic tandem asymmetric reactions constitute a powerful strategy for the asymmetric construction of nonadjacent stereocenters in acyclic molecules directly from achiral precursors. In this Communication, we report a highly enantioselective and diastereose... |
|
|
Asymmetric conjugate addition of oxindoles to 2-chloroacrylonitrile: a highly effective organocatalytic strategy for simultaneous construction of 1,3-nonadjacent stereocenters leading to chiral pyrroloindolines.
Chemistry 16(48) , 14290-4, (2010)
|
|
|
Genotoxicity of 2-halosubstituted enals and 2-chloroacrylonitrile in the Ames test and the SOS-chromotest.
Mutat. Res. 322(4) , 321-8, (1994) 2-Chloroacrolein and 2-bromoacrolein are very potent direct mutagens not requiring metabolic activation in Salmonella typhimurium strains TA 100 and TA 1535. Mutagenic activities decrease with increasing degree of methyl substitution at carbon atom C-3 of the... |
|
|
Comparative developmental toxicities of aliphatic nitriles: in vivo and in vitro observations.
Toxicol. Appl. Pharmacol. 163(2) , 149-63, (2000) The effects on embryonic development of a series of eight saturated (acetonitrile, propionitrile, and n-butyronitrile) and unsaturated (acrylonitrile, methacrylonitrile, allylnitrile, cis-2-pentenenitrile, and 2-chloroacrylonitrile) nitriles were compared in ... |
|
|
Effects of aliphatic nitriles in micromass cultures of rat embryo limb bud cells.
Toxicol. In Vitro 18(3) , 311-8, (2004) The relative effects of a series of eight saturated (acetonitrile, propionitrile and n-butyronitrile) and unsaturated (acrylonitrile, allylnitrile, methacrylonitrile, cis-2-pentenenitrile, and 2-chloroacrylonitrile) aliphatic nitriles were evaluated using an ... |
|
|
J. Org. Chem. 58 , 5200, (1993)
|
|
|
J. Chem. Soc. Chem. Commun. , 814, (1993)
|