H-D-Tyr(Me)-OH
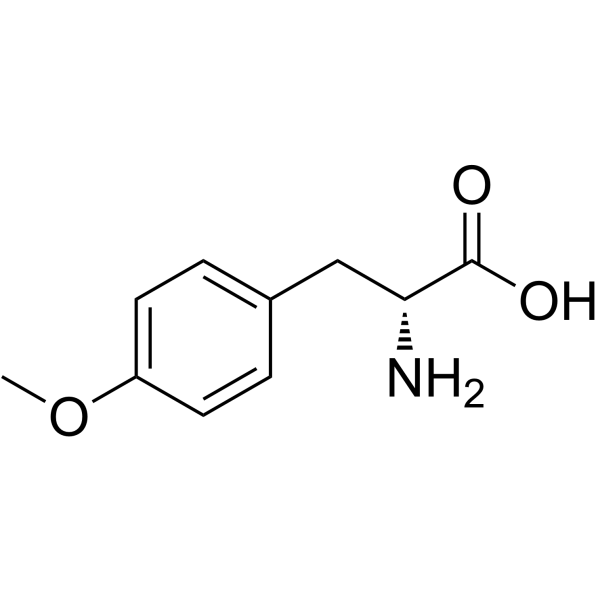
H-D-Tyr(Me)-OH structure
|
Common Name | H-D-Tyr(Me)-OH | ||
|---|---|---|---|---|
| CAS Number | 39878-65-4 | Molecular Weight | 195.215 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 350.6±32.0 °C at 760 mmHg | |
| Molecular Formula | C10H13NO3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 165.8±25.1 °C | |
|
Expanding the genetic code of Escherichia coli.
Science 292(5516) , 498-500, (2001) A unique transfer RNA (tRNA)/aminoacyl-tRNA synthetase pair has been generated that expands the number of genetically encoded amino acids in Escherichia coli. When introduced into E. coli, this pair leads to the in vivo incorporation of the synthetic amino ac... |
|
|
An expanded eukaryotic genetic code.
Science 301(5635) , 964-7, (2003) We describe a general and rapid route for the addition of unnatural amino acids to the genetic code of Saccharomyces cerevisiae. Five amino acids have been incorporated into proteins efficiently and with high fidelity in response to the nonsense codon TAG. Th... |
|
|
A genetically encoded photocaged amino acid.
J. Am. Chem. Soc. 126(44) , 14306-7, (2004) We have developed a second orthogonal tRNA/synthetase pair for use in yeast based on the Escherichia coli tRNALeu/leucyl tRNA-synthetase pair. Using a novel genetic selection, we have identified a series of synthetase mutants that selectively charge the amber... |
|
|
Stereochemical basis for engineered pyrrolysyl-tRNA synthetase and the efficient in vivo incorporation of structurally divergent non-native amino acids.
ACS Chem. Biol. 6(7) , 733-43, (2011) Unnatural amino acids (Uaas) can be translationally incorporated into proteins in vivo using evolved tRNA/aminoacyl-tRNA synthetase (RS) pairs, affording chemistries inaccessible when restricted to the 20 natural amino acids. To date, most evolved RSs aminoac... |
|
|
Two-dimensional NMR and all-atom molecular dynamics of cytochrome P450 CYP119 reveal hidden conformational substates.
J. Biol. Chem. 285(13) , 9594-603, (2010) Cytochrome P450 enzymes are versatile catalysts involved in a wide variety of biological processes from hormonal regulation and antibiotic synthesis to drug metabolism. A hallmark of their versatility is their promiscuous nature, allowing them to recognize a ... |
|
|
Nonphosphorylatable substrate analogs selectively block autophosphorylation and activation of the insulin receptor, epidermal growth factor receptor, and pp60v-src kinases.
J. Biol. Chem. 264(14) , 7831-6, (1989) The receptors for insulin and epidermal growth factor undergo tyrosine autophosphorylation in response to ligand stimulation, while pp60v-src is an unregulated tyrosine kinase. In this report we show that each of the kinases phosphorylates an exogenous peptid... |
|
|
Ligand-induced conformational heterogeneity of cytochrome P450 CYP119 identified by 2D NMR spectroscopy with the unnatural amino acid (13)C-p-methoxyphenylalanine.
J. Am. Chem. Soc. 130(48) , 16168-9, (2008) Conformational dynamics are thought to play an important role in ligand binding and catalysis by cytochrome P450 enzymes, but few techniques exist to examine them in molecular detail. Using a unique isotopic labeling strategy, we have site specifically insert... |
|
|
Structure-based design of mutant Methanococcus jannaschii tyrosyl-tRNA synthetase for incorporation of O-methyl-L-tyrosine.
Proc. Natl. Acad. Sci. U. S. A. 99(10) , 6579-84, (2002) Although incorporation of amino acid analogs provides a powerful means of producing new protein structures with interesting functions, many amino acid analogs cannot be incorporated easily by using the wild-type aminoacyl-tRNA synthetase (aaRS). To be able to... |
|
|
Reprogramming the amino-acid substrate specificity of orthogonal aminoacyl-tRNA synthetases to expand the genetic code of eukaryotic cells.
Nat. Protoc. 2(10) , 2590-600, (2007) The genetic code of living organisms has been expanded to allow the site-specific incorporation of unnatural amino acids into proteins in response to the amber stop codon UAG. Numerous amino acids have been incorporated including photo-crosslinkers, chemical ... |
|
|
Design of oxytocin antagonists, which are more selective than atosiban.
J. Pept. Sci. 7(9) , 449-65, (2001) We report the solid phase synthesis of four pairs of L- and D-thienylalanine (Thi/D-Thi) position two modified analogues of the following four oxytocin (OT) antagonists: des-9-glycinamide [1-(beta-mercapto-beta,beta-pentamethylene propionic acid), 2-O-methylt... |