| Structure | Name/CAS No. | Articles |
|---|---|---|
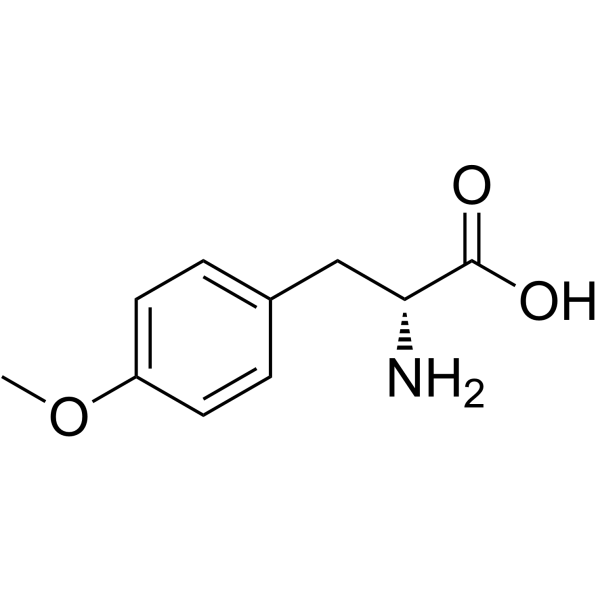 |
H-D-Tyr(Me)-OH
CAS:39878-65-4 |
|
 |
O-Methyl-L-tyrosine
CAS:6230-11-1 |