Thioxomanganese
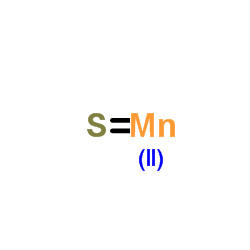
Thioxomanganese structure
|
Common Name | Thioxomanganese | ||
|---|---|---|---|---|
| CAS Number | 18820-29-6 | Molecular Weight | 87.003 | |
| Density | 3,99 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | MnS | Melting Point | 1610ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Unusual formation of single-crystal manganese sulfide microboxes co-mediated by the cubic crystal structure and shape.
Angew. Chem. Int. Ed. Engl. 51(29) , 7267-70, (2012) Kept cubic: MnS microboxes, which act as an anode material for lithium ion batteries, are synthesized by a simple H(2)S gas sulfidation approach (TEM images show porous and hollow microcubes and a microbox). The formation of the single crystals is aided by th... |
|
|
Rapid and effective labeling of brain tissue using TAT-conjugated CdS:Mn/ZnS quantum dots.
Chem. Commun. (Camb.) (25) , 3144-6, (2005) TAT (a cell penetrating peptide)-conjugated CdSratioMn/ZnS quantum dots (Qdots), intra-arterially delivered to a rat brain, rapidly (within a few minutes) labeled the brain tissue without manipulating the blood-brain-barrier (BBB). Qdot loading was sufficient... |
|
|
Carcinogenic sulfide salts of nickel and cadmium induce H2O2 formation by human polymorphonuclear leukocytes.
Cancer Res. 50(23) , 7564-70, (1990) Some derivatives of nickel, cadmium, and cobalt are carcinogenic in humans and/or animals but their mechanisms of action are not known. We show that they are capable of stimulating human polymorphonuclear leukocytes (PMNs), as measured by H2O2 formation, a kn... |
|
|
Use of a paramagnetic substance, colloidal manganese sulfide, as an NMR contrast material in rats.
J. Nucl. Med. 25(5) , 604-7, (1984) Paramagnetic pharmaceuticals ( magnetopharmaceuticals ) that are suitably distributed into specific organ systems or diseased sites might be clinically useful for tissue contrast enhancement in nuclear magnetic resonance images. To determine whether an insolu... |
|
|
The big red shift of photoluminescence of Mn dopants in strained CdS: a case study of Mn-doped MnS-CdS heteronanostructures.
J. Am. Chem. Soc. 132(19) , 6618-9, (2010) The red photoluminescence of Mn dopants in MnS-CdS heteronanostructures has been observed for the first time. The red photoluminescence at 650 nm derives from emission due to the (4)T(1) --> (6)A(1) transition of Mn(2+) dopants in a CdS matrix exposed to giga... |
|
|
Mineral-assisted pathways in prebiotic synthesis: photoelectrochemical reduction of carbon(+IV) by manganese sulfide.
J. Am. Chem. Soc. 126(36) , 11247-53, (2004) Photoelectrochemistry on mineral surfaces has the potential to play a central role in the prebiotic syntheses of building blocks for biomolecules. In this study, photoreduction of C(+IV) as bicarbonate is used as a probe to investigate the photoelectrochemica... |
|
|
Hydrothermal synthesis of a doped Mn-Cd-S solid solution as a visible-light-driven photocatalyst for H2 evolution.
ChemSusChem 4(2) , 269-73, (2011) The effect of metal doping (i.e., with Cr, Fe, Ni, Cu, Zn, Ag and Sn) on the crystal structure of hydrothermally synthesized Mn(1-x)Cd(x) S (where x≈0.1) is studied with the aim of enhancing photocatalytic activity. In contrast to the low-crystalline, undoped... |
|
|
Manganese sulfide formation via concomitant microbial manganese oxide and thiosulfate reduction.
Environ. Microbiol. 13(12) , 3275-88, (2011) The dissimilatory metal-reducing bacterium, Shewanella oneidensis MR-1 produced γ-MnS (rambergite) nanoparticles during the concurrent reduction of MnO₂ and thiosulfate coupled to H₂ oxidation. To investigate effect of direct microbial reduction of MnO₂ on Mn... |
|
|
A facile route for the synthesis of poly(N-vinylcarbazole)/manganese sulphide quantum dots nanocomposites with enhanced optical properties.
J. Nanosci. Nanotechnol. 8(11) , 6031-7, (2008) This communication describes a facile route for the synthesis of manganese sulphide (MnS) quantum dots (QDs) contained in poly(N-vinyl carbazole) (PNVC) nanocomposites. MnS QDs were synthesized using the single-source precursor method by thermolysing tetramet... |
|
|
Hydrothermal Synthesis of Pure r-Phase Manganese(II) Sulfide without the Use of Organic Reagents Michel FM, et al.
Chem. Mater. 18 , 1726-1736, (2006)
|