Oxaprozin
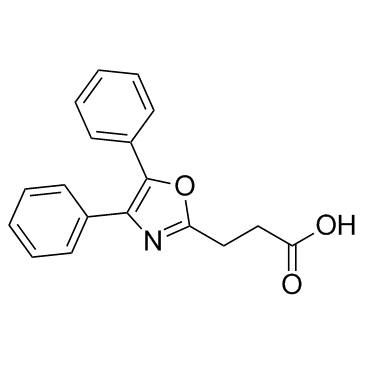
Oxaprozin structure
|
Common Name | Oxaprozin | ||
|---|---|---|---|---|
| CAS Number | 21256-18-8 | Molecular Weight | 293.317 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 467.0±33.0 °C at 760 mmHg | |
| Molecular Formula | C18H15NO3 | Melting Point | 154ºC | |
| MSDS | Chinese USA | Flash Point | 236.2±25.4 °C | |
|
Potential therapeutic approach to SAPHO.
Semin. Arthritis Rheum. 29(5) , 332-4, (2000)
|
|
|
Analgesic and Anti-Inflammatory Effects of Oxaprozin and Naproxen Sodium After Removal of Impacted Lower Third Molars: A Randomized, Double-Blind, Placebo-Controlled Crossover Study
J. Oral Maxillofac. Surg. 68(5) , 1018-24, (2010) Purpose In this study, oxaprozin, a long-acting nonsteroidal anti-inflammatory drug, and naproxen sodium were compared in terms of their effects on edema, pain, and trismus after surgery for impacted mandibular third molars. |
|
|
Oxaprozin versus diclofenac in NSAID-refractory periarthritis pain of the shoulder.
Curr. Med. Res. Opin. 20(8) , 1279-90, (2004) To evaluate the efficacy and safety of oxaprozin in comparison with diclofenac in patients with periarthritis pain of the shoulder previously unsuccessfully treated with nonsteroidal anti-inflammatory drugs other than diclofenac and oxaprozin.In this open, mu... |
|
|
LC method for the quantitative determination of oxaprozin and its impurities in the bulk drug.
J. Pharm. Biomed. Anal. 22(4) , 651-9, (2000) A reversed phase linear gradient liquid chromatographic method was developed for the separation and quantitative determination of the seven known process related impurities and one degraded product of oxaprozin in the bulk drug material. An Inertsil-ODS 3V (1... |
|
|
Effects of Transcutol P on the corneal permeability of drugs and evaluation of its ocular irritation of rabbit eyes.
J. Pharm. Pharmacol. 58(1) , 45-50, (2006) Our purpose was to explore the use of Transcutol P (Trans) in an ocular drug delivery system. The effect of Trans on the corneal permeability of drugs was investigated in-vitro, using isolated rabbit corneas. The ocular irritation of Trans was also tested in ... |
|
|
Development of a new delivery system consisting in 'drug-in cyclodextrin-in PLGA nanoparticles'.
J. Microencapsul. 27(6) , 479-86, (2010) A combined approach based on drug cyclodextrin (CD) complexation and loading into PLGA nanoparticles (NP) has been developed to improve oxaprozin therapeutic efficiency. This strategy exploits the solubilizing and stabilizing properties of CDs and the prolong... |
|
|
A review of the emerging profile of the anti-inflammatory drug oxaprozin.
Expert Opin. Pharmacother. 6(5) , 777-85, (2005) Oxaprozin is a nonsteroidal anti-inflammatory drug characterised by a propionic acid-based structure. It is able to diffuse easily into inflamed synovial tissues after oral administration. Although discovered > 20 years ago, it is now under intensive investig... |
|
|
Analytic performance evaluation of a new turbidimetric immunoassay for phenytoin on the ADVIA 1650 analyzer: effect of phenytoin metabolite and analogue.
Ther. Drug Monit. 27(3) , 305-8, (2005) Phenytoin is an anticonvulsant that requires therapeutic drug monitoring. Recently, Bayer HealthCare, Diagnostics Division released a turbidimetric immunoassay of phenytoin on the ADVIA 1650 analyzer. We evaluated the analytic performance of this assay by com... |
|
|
Synthesis, characterization and antiproliferative activity of transition metal complexes with 3-(4,5-diphenyl-1,3-oxazol-2-yl)propanoic acid (oxaprozin).
Chem. Pharm. Bull. 60(7) , 865-9, (2012) A series of novel Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) complexes with oxaprozin (Hoxa), a non-steroidal anti-inflammatory drug, has been synthesized. The drug and complexes have been characterized by elemental and thermogravimetric (TG) analysis, Fourier... |
|
|
Effects of isopropyl palmitate on the skin permeation of drugs.
Biol. Pharm. Bull. 29(11) , 2324-6, (2006) The model penetrants oxaprozin, nimesulide, gliclazide, and ribavirin, because of their different lipophilicities, were selected to assess the enhancing activity of pre-treatment solutions consisting of isopropyl palmitate (IP) in ethanol (5%, 10%, 15%and 20%... |