1-Chloro-2-octyne
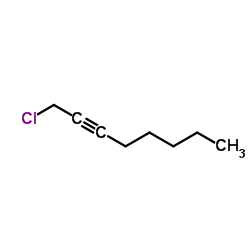
1-Chloro-2-octyne structure
|
Common Name | 1-Chloro-2-octyne | ||
|---|---|---|---|---|
| CAS Number | 51575-83-8 | Molecular Weight | 144.642 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 190.6±13.0 °C at 760 mmHg | |
| Molecular Formula | C8H13Cl | Melting Point | N/A | |
| MSDS | USA | Flash Point | 64.6±15.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Unprecedented Spectroscopic and Computational Evidence for Allenyl and Propargyl Titanocene(IV) Complexes: Electrophilic Quenching of Their Metallotropic Equilibrium.
Chemistry 22 , 2427-39, (2016) The synthesis and structural characterization of allenyl titanocene(IV) [TiClCp2 (CH=C=CH2 )] 3 and propargyl titanocene(IV) [TiClCp2 (CH2 -C≡C-(CH2 )4 CH3 )] 9 have been described for the first time. Advanced NMR methods including diffusion NMR methods (diff... |
|
|
Nickel-catalyzed substitution reactions of propargyl halides with organotitanium reagents.
Org. Biomol. Chem. 12(38) , 7634-42, (2014) A simple and mild catalytic coupling reaction of propargyl halides with organotitanium reagents is reported. The reaction of propargyl bromide with organo-titanium reagents mediated by NiCl2 (2 mol%) and PCy3 (4 mol%) in CH2Cl2 afforded coupling product allen... |
|
|
A new method for the preparation of 1,5-diynes. Synthesis of (4E,6Z,10Z)-4,6,10-hexadecatrien-1-ol, the pheromone component of the cocoa pod borer moth Conopomorpha cramerella.
J. Org. Chem. 70(7) , 2594-7, (2005) [reaction: see text] A new method for the synthesis of 1,5-diynes, from the reaction of 1,3-dilithiopropyne and propargyl chlorides, was developed. This new methodology was used to prepare (4E,6Z,10Z)-4,6,10-hexadecatrien-1-ol, one of the pheromone components... |
|
|
The Dielectric Properties of Acetylenic Compounds. V. Acetylenic Halides and Alcohols. Toussaint JA and Wenzke HH.
J. Am. Chem. Soc. 57(4) , 668-70, (1935)
|
|
|
Palladium-Catalyzed Regioselective Coupling of Propargylic Substrates with Terminal Alkynes. Application to the Synthesis of 1, 2-Dien-4-ynes. Condon-Gueugnot S and Linstrumelle G.
Tetrahedron 56(13) , 1851-57, (2000)
|