docetaxel trihydrate
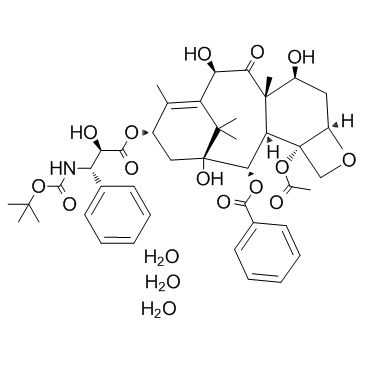
docetaxel trihydrate structure
|
Common Name | docetaxel trihydrate | ||
|---|---|---|---|---|
| CAS Number | 148408-66-6 | Molecular Weight | 861.925 | |
| Density | 1.37 g/cm3 | Boiling Point | 1016.9ºC at 760 mmHg | |
| Molecular Formula | C43H59NO17 | Melting Point | 186-192ºC | |
| MSDS | N/A | Flash Point | 568.8ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Losartan competitively inhibits CYP2C8-dependent paclitaxel metabolism in vitro.
Biol. Pharm. Bull. 37(9) , 1550-4, (2014) The present study aimed to characterize the inhibitory effects of losartan, an angiotensin II receptor blocker, on CYP2C8. Inhibition experiments were based on human lymphoblast-expressed recombinant CYP2C8 (rCYP2C8) and paclitaxel as a CYP2C8 substrate. The ... |
|
|
The potential of anti-vascular endothelial growth factor therapy in metastatic breast cancer: clinical experience with anti-angiogenic agents, focusing on bevacizumab.
Eur. J. Cancer 44(7) , 912-20, (2008) The importance of angiogenesis in tumour growth and development is well known. Overexpression of vascular endothelial growth factor (VEGF), the key mediator of angiogenesis, is associated with poor prognosis in breast cancer. As a result, several therapeutic ... |
|
|
Advancing bioluminescence imaging technology for the evaluation of anticancer agents in the MDA-MB-435-HAL-Luc mammary fat pad and subrenal capsule tumor models.
Clin. Cancer Res. 15(1) , 238-46, (2009) Tumors grafted s.c. or under the mammary fat pad (MFP) rarely develop efficient metastasis. By applying bioluminescence imaging (BLI) technology, the MDA-MB-435-HAL-Luc subrenal capsule (SRC) model was compared with the MFP model for disease progression, meta... |
|
|
Bevacizumab/docetaxel association is more efficient than docetaxel alone in reducing breast and prostate cancer cell growth: A new paradigm for understanding the therapeutic effect of combined treatment
Eur. J. Cancer 46 , 3022-36, (2010) Bevacizumab (Bvz), a Vascular Endothelial Growth Factor (VEGF)-targeted humanised monoclonal antibody, provides clinical benefit in combination with docetaxel (DXL), a microtubule-stabilising agent, in the treatment of metastatic breast and prostate cancers. ... |
|
|
Novel therapeutic strategies in development for prostate cancer.
Expert Opin. Investig. Drugs 17(1) , 13-22, (2008) Relatively few therapy options exist for patients with prostate cancer that has become resistant to androgen-deprivation therapy and has metastasized to distant sites. Survival for such patients is poor with a median survival of approximately 20 months from t... |
|
|
Treatment of metastatic breast cancer: looking towards the future.
Breast Cancer Res. Treat. 114(3) , 413-22, (2009) The armamentarium for the treatment of metastatic breast cancer is increasing with the introduction of newer chemotherapeutic agents and the development of molecular targeted therapies. The clinical utility of anthracyclines in advanced breast cancer has been... |
|
|
Phase 2 study of carboplatin, docetaxel, and bevacizumab as frontline treatment for advanced nonsmall-cell lung cancer.
Cancer 116(10) , 2401-8, (2010) Bevacizumab has recently been demonstrated to prolong overall survival when added to carboplatin and paclitaxel for chemotherapy-naïve patients with nonsquamous nonsmall-cell lung cancer (NSCLC). However, the effects of combining bevacizumab with other standa... |
|
|
Bevacizumab plus 5-fluorouracil induce growth suppression in the CWR-22 and CWR-22R prostate cancer xenografts.
Mol. Cancer Ther. 6(8) , 2149-57, (2007) Prostate cancer is the most common malignancy in men. Although patients with metastatic prostate cancer can benefit from androgen ablation, most of them will die of prostate cancer progression to an androgen-refractory state. In the present study, the effects... |
|
|
Reversal of docetaxel resistance with bevacizumab and thalidomide.
Clin. Genitourin. Cancer 7(2) , E37-8, (2009) Taxane resistance is a common clinical problem in the treatment of many metastatic malignancies. Observational clues or evidence of overcoming such resistance is important for developing treatments that can extend taxane activity. We report a case of the reve... |
|
|
A phase II trial of docetaxel with bevacizumab as first-line therapy for HER2-negative metastatic breast cancer (TORI B01).
Clin. Breast Cancer 10(4) , 307-12, (2010) Addition of the antiangiogenic agent bevacizumab to paclitaxel significantly improves response rates and progression-free survival for metastatic breast cancer (MBC). To assess the activity of docetaxel plus bevacizumab, a multicenter phase II trial was condu... |