Diamine Oxidase from porcine kidney
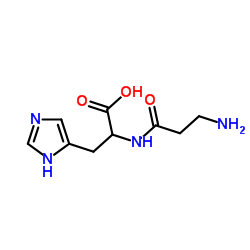
Diamine Oxidase from porcine kidney structure
|
Common Name | Diamine Oxidase from porcine kidney | ||
|---|---|---|---|---|
| CAS Number | 9001-53-0 | Molecular Weight | 226.232 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 656.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C9H14N4O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 350.7±31.5 °C | |
|
Diamine oxidase from pig kidney: new purification method and amino acid composition.
Prep. Biochem. 12(1) , 11-28, (1982) Several methods for the isolation of apparently homogeneous pig kidney diamine oxidase have been reported in recent years (1-7), but these procedures allow to obtain only little amounts of material making very difficult the study of the molecular properties o... |
|
|
The glycoprotein nature of pig kidney diamine oxidase. Role of disulphide groups and arginine residues in the concanavalin A-diamine oxidase interaction.
Biochem. J. 253(1) , 103-7, (1988) Pig kidney diamine oxidase (DAO) was found to contain 5% (w/w) natural hexose, 3.25% glucosamine, 2.61% N-acetylglucosamine and 0.25% N-acetylneuraminic acid. The enzyme exhibited strong affinity towards concanavalin A (Con A) with a stoichiometry of 1:4.6. T... |
|
|
A luminescence-based test for determining ornithine decarboxylase activity.
Anal. Biochem. 287 , 299-302, (2000) A sensitive chemiluminescence-based method for the assay of ornithine decarboxylase (ODC) has been developed. This method, which permits the detection of putrescine (the product of ODC) at a picomolar range, can be used to determine ODC activity in cellular e... |
|
|
N-linked oligosaccharide structures in the diamine oxidase from porcine kidney.
Carbohydr. Res. 323 , 111-25, (2000) Structures of the N-linked glycans released from porcine kidney diamine oxidase (DAO) were characterized utilizing various analytical techniques, including matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI/TOF-MS), high-perfo... |
|
|
Diamine oxidase and catalase are expressed in the same cells but are present in different subcellular compartments in porcine kidney.
Inflamm. Res. 48 , s81-2, (1999)
|
|
|
Evolution of a constitutional dynamic library driven by self-organisation of a helically folded molecular strand.
Chemistry 16(16) , 4903-10, (2010) Conversion of macrocyclic imine entities into helical strands was achieved through three- and four-component exchange reactions within constitutionally dynamic libraries. The generation of sequences of the intrinsic helicity codon, based on the hydrazone-pyri... |
|
|
Taylor, H., and Tabor, C. W.
Meth. Enzymol. 17B , 735-740
|
|
|
Dextranase in sugar industry: A review Efraín Rodríguez Jiménez
Sugar tech 11(2) , 124-134, (2009)
|