Diamine oxidase from pig kidney: new purification method and amino acid composition.
A Rinaldi, P Vecchini, G Floris
Index: Prep. Biochem. 12(1) , 11-28, (1982)
Full Text: HTML
Abstract
Several methods for the isolation of apparently homogeneous pig kidney diamine oxidase have been reported in recent years (1-7), but these procedures allow to obtain only little amounts of material making very difficult the study of the molecular properties of the enzyme. Drawing useful indication from the purification procedures previously reported, we were able to set up a new method which allows to obtain homogeneous enzyme samples in high yield and with good reproducibility. This procedure allowed to determine with greater accuracy the molecular weight of the enzyme that resulted to be 170,000 daltons by gel chromatography and 145,000 by ultracentrifuge. The enzyme is composed of two apparently identical subunits and contains two copper atoms per dimer. The amino acid composition of the protein has been also worked out and found similar to those already reported for other copper dependent amine oxidases. Pig kidney diamine oxidase is a glycoprotein containing about 20% sugars by weight.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
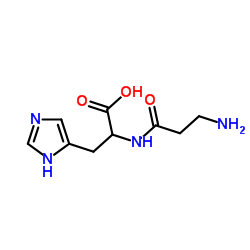 |
Diamine Oxidase from porcine kidney
CAS:9001-53-0 |
C9H14N4O3 |
|
The glycoprotein nature of pig kidney diamine oxidase. Role ...
1988-07-01 [Biochem. J. 253(1) , 103-7, (1988)] |
|
A luminescence-based test for determining ornithine decarbox...
2000-12-15 [Anal. Biochem. 287 , 299-302, (2000)] |
|
N-linked oligosaccharide structures in the diamine oxidase f...
2000-01-12 [Carbohydr. Res. 323 , 111-25, (2000)] |
|
Diamine oxidase and catalase are expressed in the same cells...
1999-04-01 [Inflamm. Res. 48 , s81-2, (1999)] |
|
Evolution of a constitutional dynamic library driven by self...
2010-04-26 [Chemistry 16(16) , 4903-10, (2010)] |