7-Azaindole
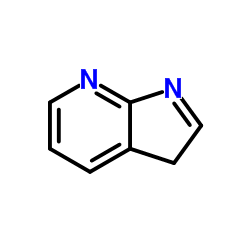
7-Azaindole structure
|
Common Name | 7-Azaindole | ||
|---|---|---|---|---|
| CAS Number | 271-63-6 | Molecular Weight | 118.14 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 283.1±40.0 °C at 760 mmHg | |
| Molecular Formula | C7H6N2 | Melting Point | 105-107 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 125.0±27.3 °C | |
|
Selectivity of kinase inhibitor fragments.
J. Med. Chem. 54 , 5131-43, (2011) A kinase-focused screening set of fragments has been assembled and has proved successful for the discovery of ligand-efficient hits against many targets. Here we present some of our general conclusions from this exercise. Notably, we present the first profili... |
|
|
Synthesis of 7-azaserotonin: its photophysical properties associated with excited state proton transfer reaction.
J. Am. Chem. Soc. 128 , 14426, (2006) We report the synthesis of 3-(2-aminoethyl)-5-ol-1H-pyrrolo[2,3-b]pyridine (7-azaserotonin), which may potentially serve as an agonist or antagonist of serotonin receptors. In alcohols, the solvent (e.g., ethanol) catalyzed proton-transfer reaction takes plac... |
|
|
A practical synthesis of 2-((1H-pyrrolo[2,3-b]pyridine-4-yl)methylamino)-5- fluoronicotinic acid.
J. Org. Chem. 71 , 4021-4023, (2006) A practical synthesis of a key pharmaceutical intermediate, 2-[(1H-pyrrolo[2,3-b]pyridine-4-yl)methylamino]-5-fluoronicotinic acid (1), is described. To introduce the aminomethyl moiety of 2 via a palladium-catalyzed cyanation/reduction sequence, a regioselec... |
|
|
Identification of 4-(4-aminopiperidin-1-yl)-7H-pyrrolo[2,3-d]pyrimidines as selective inhibitors of protein kinase B through fragment elaboration.
J. Med. Chem. 51 , 2147-57, (2008) Fragment-based screening identified 7-azaindole as a protein kinase B inhibitor scaffold. Fragment elaboration using iterative crystallography of inhibitor-PKA-PKB chimera complexes efficiently guided improvements in the potency and selectivity of the compoun... |
|
|
Rapid evolution of 6-phenylpurine inhibitors of protein kinase B through structure-based design.
J. Med. Chem. 50 , 2289-92, (2007) 6-phenylpurines were identified as novel, ATP-competitive inhibitors of protein kinase B (PKB/Akt) from a fragment-based screen and were rapidly progressed to potent compounds using iterative protein-ligand crystallography with a PKA-PKB chimeric protein. An ... |