| Structure | Name/CAS No. | Articles |
|---|---|---|
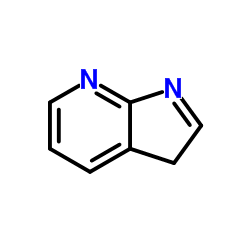 |
7-Azaindole
CAS:271-63-6 |
Xin Wang, Ben Zhi, Jean Baum, Ying Chen, Richard Crockett, Liang Huang, Shawn Eisenberg, John Ng, Robert Larsen, Mike Martinelli, Paul Reider
Index: J. Org. Chem. 71 , 4021-4023, (2006)
Full Text: HTML
A practical synthesis of a key pharmaceutical intermediate, 2-[(1H-pyrrolo[2,3-b]pyridine-4-yl)methylamino]-5-fluoronicotinic acid (1), is described. To introduce the aminomethyl moiety of 2 via a palladium-catalyzed cyanation/reduction sequence, a regioselective chlorination of 7-azaindole via the N-oxide was developed. A highly selective monodechlorination of 2,6-dichloro-5-fluoronicotinic acid was discovered to afford the nicotinic acid 3. The two building blocks 2 and 3 were then coupled to complete the preparation of 1.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
7-Azaindole
CAS:271-63-6 |
C7H6N2 |
|
Selectivity of kinase inhibitor fragments.
2011-07-28 [J. Med. Chem. 54 , 5131-43, (2011)] |
|
Synthesis of 7-azaserotonin: its photophysical properties as...
2006-11-15 [J. Am. Chem. Soc. 128 , 14426, (2006)] |
|
Identification of 4-(4-aminopiperidin-1-yl)-7H-pyrrolo[2,3-d...
2008-04-10 [J. Med. Chem. 51 , 2147-57, (2008)] |
|
Rapid evolution of 6-phenylpurine inhibitors of protein kina...
2007-05-17 [J. Med. Chem. 50 , 2289-92, (2007)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved