N-alpha-Acetyl-L-ornithine
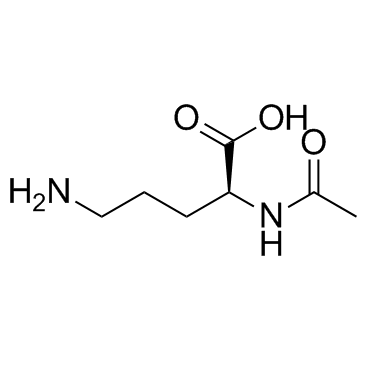
N-alpha-Acetyl-L-ornithine structure
|
Common Name | N-alpha-Acetyl-L-ornithine | ||
|---|---|---|---|---|
| CAS Number | 6205-08-9 | Molecular Weight | 174.19800 | |
| Density | 1.171g/cm3 | Boiling Point | 436.2ºC at 760 mmHg | |
| Molecular Formula | C7H14N2O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 217.6ºC | |
|
Metabolomic profiling of serum in the progression of Alzheimer's disease by capillary electrophoresis-mass spectrometry.
Electrophoresis 35(23) , 3321-30, (2014) There is high interest in the discovery of early diagnostic biomarkers of Alzheimer's disease, for which metabolomics exhibits a great potential. In this work, a metabolomic approach based on ultrafiltration and analysis by CE-MS has been used to obtain repre... |
|
|
Inhibitors of N(alpha)-acetyl-L-ornithine deacetylase: synthesis, characterization and analysis of their inhibitory potency.
Amino Acids 38 , 1155-1164, (2010) A series of N (alpha)-acyl (alkyl)- and N (alpha)-alkoxycarbonyl-derivatives of L- and D-ornithine were prepared, characterized, and analyzed for their potency toward the bacterial enzyme N (alpha)-acetyl-L-ornithine deacetylase (ArgE). ArgE catalyzes the con... |
|
|
Model-driven discovery of underground metabolic functions in Escherichia coli.
Proc. Natl. Acad. Sci. U. S. A. 112(3) , 929-34, (2015) Enzyme promiscuity toward substrates has been discussed in evolutionary terms as providing the flexibility to adapt to novel environments. In the present work, we describe an approach toward exploring such enzyme promiscuity in the space of a metabolic networ... |
|
|
Crystal structure of N-acetylornithine transcarbamylase from Xanthomonas campestris: a novel enzyme in a new arginine biosynthetic pathway found in several eubacteria.
J. Biol. Chem. 280 , 14366-14369, (2005) We have identified in Xanthomonas campestris a novel N-acetylornithine transcarbamylase that replaces ornithine transcarbamylase in the canonic arginine biosynthetic pathway of several Eubacteria. The crystal structures of the protein in the presence and abse... |
|
|
Structures of N-acetylornithine transcarbamoylase from Xanthomonas campestris complexed with substrates and substrate analogs imply mechanisms for substrate binding and catalysis.
Proteins 64 , 532-542, (2006) N-acetyl-L-ornithine transcarbamoylase (AOTCase) is a new member of the transcarbamoylase superfamily that is essential for arginine biosynthesis in several eubacteria. We report here crystal structures of the binary complexes of AOTCase with its substrates, ... |
|
|
Reversible post-translational carboxylation modulates the enzymatic activity of N-acetyl-L-ornithine transcarbamylase.
Biochemistry 49 , 6887-6895, (2010) N-Acetyl-l-ornithine transcarbamylase (AOTCase), rather than ornithine transcarbamylase (OTCase), is the essential carbamylase enzyme in the arginine biosynthesis of several plant and human pathogens. The specificity of this unique enzyme provides a potential... |
|
|
Both the Jasmonic Acid and the Salicylic Acid Pathways Contribute to Resistance to the Biotrophic Clubroot Agent Plasmodiophora brassicae in Arabidopsis.
Plant Cell Physiol. 56 , 2158-68, (2015) The role of salicylic acid (SA) and jasmonic acid (JA) signaling in resistance to root pathogens has been poorly documented. We assessed the contribution of SA and JA to basal and partial resistance of Arabidopsis to the biotrophic clubroot agent Plasmodiopho... |