Crystal structure of N-acetylornithine transcarbamylase from Xanthomonas campestris: a novel enzyme in a new arginine biosynthetic pathway found in several eubacteria.
Dashuang Shi, Hiroki Morizono, Xiaolin Yu, Lauren Roth, Ljubica Caldovic, Norma M Allewell, Michael H Malamy, Mendel Tuchman
Index: J. Biol. Chem. 280 , 14366-14369, (2005)
Full Text: HTML
Abstract
We have identified in Xanthomonas campestris a novel N-acetylornithine transcarbamylase that replaces ornithine transcarbamylase in the canonic arginine biosynthetic pathway of several Eubacteria. The crystal structures of the protein in the presence and absence of the reaction product, N-acetylcitrulline, were determined. This new family of transcarbamylases lacks the DxxSMG motif that is characteristic of all ornithine transcarbamylases (OTCases) and contains a novel proline-rich loop that forms part of the active site. The specificity for N-acetylornithine is conferred by hydrogen bonding with residues in the proline-rich loop via water molecules and by hydrophobic interactions with residues from the adjacent 80's, 120's, and proline-rich loops. This novel protein structure provides a starting point for rational design of specific analogs that may be useful in combating human and plant pathogens that utilize acetylornithine transcarbamylase rather than ornithine transcarbamylase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
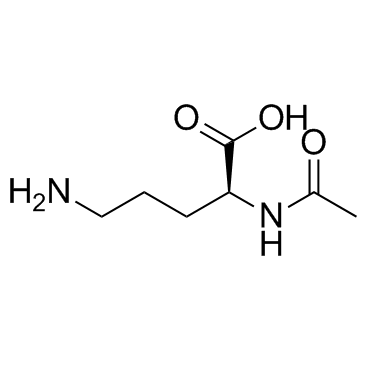 |
N-alpha-Acetyl-L-ornithine
CAS:6205-08-9 |
C7H14N2O3 |
|
Metabolomic profiling of serum in the progression of Alzheim...
2014-12-01 [Electrophoresis 35(23) , 3321-30, (2014)] |
|
Inhibitors of N(alpha)-acetyl-L-ornithine deacetylase: synth...
2010-04-01 [Amino Acids 38 , 1155-1164, (2010)] |
|
Model-driven discovery of underground metabolic functions in...
2015-01-20 [Proc. Natl. Acad. Sci. U. S. A. 112(3) , 929-34, (2015)] |
|
Structures of N-acetylornithine transcarbamoylase from Xanth...
2006-08-01 [Proteins 64 , 532-542, (2006)] |
|
Reversible post-translational carboxylation modulates the en...
2010-08-17 [Biochemistry 49 , 6887-6895, (2010)] |