2,6-Dichloropurine
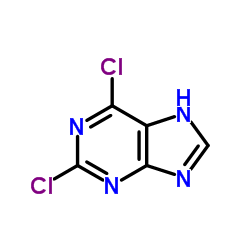
2,6-Dichloropurine structure
|
Common Name | 2,6-Dichloropurine | ||
|---|---|---|---|---|
| CAS Number | 5451-40-1 | Molecular Weight | 189.00 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 405.4±27.0 °C at 760 mmHg | |
| Molecular Formula | C5H2Cl2N4 | Melting Point | 185-195 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 231.3±9.3 °C | |
|
An efficient synthesis of 2-substituted 6-methylpurine bases and nucleosides by Fe- or Pd-catalyzed cross-coupling reactions of 2,6-dichloropurines.
J. Org. Chem. 68(14) , 5773-6, (2003) Fe-catalyzed cross-coupling reactions of 9-substituted or protected 2,6-dichloropurines with 1 equiv of methylmagnesium chloride gave regioselectively 2-chloro-6-methylpurines in good yields. The same reactions with 3 equiv of methylmagnesium chloride or Pd-c... |
|
|
Occupational contact sensitization to 2,6-dichloropurine.
Contact Dermatitis 7(6) , 349-50, (1981)
|
|
|
Occupational contact sensitization to 2,6-dichloropurine.
Contact Dermatitis 7(3) , 162-3, (1981)
|
|
|
Two tautomeric polymorphs of 2,6-dichloropurine.
Acta Crystallogr. C 67(Pt 12) , o484-6, (2011) Two polymorphs of 2,6-dichloropurine, C(5)H(2)Cl(2)N(4), have been crystallized and identified as the 9H- and 7H-tautomers. Despite differences in the space group and number of symmetry-independent molecules, they exhibit similar hydrogen-bonding motifs. Both... |
|
|
Method for the synthesis of uric acid derivatives.
Nucleosides Nucleotides Nucleic Acids 19(7) , 1193-203, (2000) A general procedure to obtain tetra-substituted uric acid by stepwise N-alkylation is described. 2,6-Dichloropurine (1) was condensed with 1-propanol by Mitsunobu reaction to give 9-propyl congener (2). Treatment of 2 with ammonia gave adenine derivative (4a)... |
|
|
A class of novel conjugates of substituted purine and Gly-AA-OBzl: synthesis and evaluation of orally analgesic activity.
Bioorg. Med. Chem. Lett. 20 , 6157-60, (2010) Aimed at the chemotherapy of chronic pain two kinds of analgesic pharmacophores, substituted purine and Gly-AA-OBzl, were coupled via a five-step-reaction procedure and 19 novel conjugates N-[2-chloro-9-(tetrahydropyran-2-yl)-9H-purin-6-yl]-N-cyclopropylglycy... |
|
|
Synthesis , 3515, (2006)
|