Method for the synthesis of uric acid derivatives.
T Maruyama, S Kozai, F Sasaki
Index: Nucleosides Nucleotides Nucleic Acids 19(7) , 1193-203, (2000)
Full Text: HTML
Abstract
A general procedure to obtain tetra-substituted uric acid by stepwise N-alkylation is described. 2,6-Dichloropurine (1) was condensed with 1-propanol by Mitsunobu reaction to give 9-propyl congener (2). Treatment of 2 with ammonia gave adenine derivative (4a), which was converted to the 8-oxoadenine (5b) in 3 steps. Methylation of 5b proceeded site-specifically to give 6-amino-2-chloro-7,8-dihydro-7-methyl-9-propylpurin-8-one (6) as a sole product. Compound 6 was successively treated with NaNO2 and iodomethane to give 2-chloro-1,6,7,8-tetrahydro-1,7-dimethyl-9-propylpurin-6,8-dione (9) accompanied by the O6-methyl product (8) in 75% and 6.9%, respectively. After nucleophilic substitution of 9 with NaOAc, the product (11) was reacted with iodomethane to give the uric acid (12) and the 2-methoxy product (13) in 46% and 15.5%, respectively. However, the reaction of 11 with the benzylating agents gave only O-benzyl products (14a,b).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
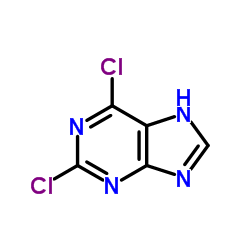 |
2,6-Dichloropurine
CAS:5451-40-1 |
C5H2Cl2N4 |
|
An efficient synthesis of 2-substituted 6-methylpurine bases...
2003-07-11 [J. Org. Chem. 68(14) , 5773-6, (2003)] |
|
Occupational contact sensitization to 2,6-dichloropurine.
1981-11-01 [Contact Dermatitis 7(6) , 349-50, (1981)] |
|
Occupational contact sensitization to 2,6-dichloropurine.
1981-05-01 [Contact Dermatitis 7(3) , 162-3, (1981)] |
|
Two tautomeric polymorphs of 2,6-dichloropurine.
2011-12-01 [Acta Crystallogr. C 67(Pt 12) , o484-6, (2011)] |
|
A class of novel conjugates of substituted purine and Gly-AA...
2010-10-15 [Bioorg. Med. Chem. Lett. 20 , 6157-60, (2010)] |