3-Methyl-2-buten-1-ol
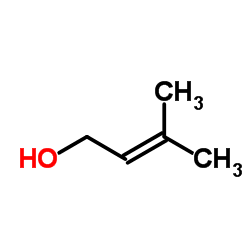
3-Methyl-2-buten-1-ol structure
|
Common Name | 3-Methyl-2-buten-1-ol | ||
|---|---|---|---|---|
| CAS Number | 556-82-1 | Molecular Weight | 86.132 | |
| Density | 0.848 | Boiling Point | 140 ºC | |
| Molecular Formula | C5H10O | Melting Point | -86ºC | |
| MSDS | Chinese USA | Flash Point | 43 ºC | |
| Symbol |


GHS02, GHS07 |
Signal Word | Warning | |
|
Rapid high performance liquid chromatography-high resolution mass spectrometry methodology for multiple prenol lipids analysis in zebrafish embryos.
J. Chromatogr. A. 1412 , 59-66, (2015) The analysis of lipid molecules in living organism is an important step in deciphering metabolic pathways. Recently, the zebrafish has been adopted as a valuable animal model system to perform in vivo metabolomics studies, however limited methodologies and pr... |
|
|
Fumigant toxicity and acetylcholinesterase inhibitory activity of 4 Asteraceae plant essential oils and their constituents against Japanese termite (Reticulitermes speratus Kolbe).
Pestic. Biochem. Physiol. 113 , 55-61, (2014) This study investigated the fumigant toxicity of 4 Asteraceae plant essential oils and their constituents against the Japanese termite Reticulitermes speratus Kolbe. Fumigant toxicity varied with plant essential oils or constituents, exposure time, and concen... |
|
|
A strategy for position-selective epoxidation of polyprenols.
J. Am. Chem. Soc. 130(25) , 8089-93, (2008) An effective strategy has been developed for the efficient site-selective epoxidation of poylolefinic isoprenoid alcohols, based on the use of an internal control element for intramolecular reaction. The approach is illustrated by application to a series of p... |
|
|
Heteromeric geranyl diphosphate synthase from mint: construction of a functional fusion protein and inhibition by bisphosphonate substrate analogs.
Arch. Biochem. Biophys. 422(1) , 52-60, (2004) Geranyl diphosphate synthase catalyzes the condensation of dimethylallyl diphosphate (C(5)) with isopentenyl diphosphate (C(5)) to produce geranyl diphosphate (C(10)), the essential precursor of monoterpenes. The enzyme from peppermint and spearmint (Menthaxp... |
|
|
Antibacterial/antifungal activity and synergistic interactions between polyprenols and other lipids isolated from Ginkgo biloba L. leaves.
Molecules 18(2) , 2166-82, (2013) Polyprenols separated from lipids are promising new components from Ginkgo biloba L. leaves (GBL). In this paper, ginkgo lipids were isolated by extraction with petroleum ether, saponification, and molecular distillation. Eight known compounds: isophytol (1),... |
|
|
Polyprenols from the needles ofTaxus chinensisvar.mairei
Fitoterapia 83(5) , 831-7, (2012) Polyprenols with various pharmacological activities were first isolated from Taxus chinensis var. mairei, which is native to China, and were identified by a high performance liquid chromatography/mass spectrometry (HPLC/MS), 1H nuclear magnetic resonance (1H ... |
|
|
Alloprenols: novel alpha-trans-polyprenols of Allophylus caudatus.
Chem. Phys. Lipids 147(2) , 103-12, (2007) A novel type of polyprenols, alloprenols, with an alpha-trans-isoprenoid unit was found in the leaves of Allophylus caudatus (Sapindaceae) besides typical alpha-cis-polyprenols. The polyprenol family (Prenol-11-13, Prenol-12 dominating) was accompanied by tra... |
|
|
Polyprenyl lipid synthesis in mammalian cells expressing human cis-prenyl transferase.
Biochem. Biophys. Res. Commun. 331(2) , 379-83, (2005) The level of cis-prenyl transferase activity has been implicated in controlling the level of biosynthesis of dolichol and dolichol intermediates. In this study, we isolated a cDNA encoding a human CPT (GenBank Accession No. ), which had substantial homology t... |
|
|
Precise bacterial polyprenol length control fails in Saccharomyces cerevisiae.
Biopolymers 86(2) , 155-64, (2007) A comparison of amino acid sequences of yeast Rer2p and Srt1p Z-prenyltransferases shows that the spatial organization of their substrate tunnels agrees with that determined by X-ray for the E. coli undecaprenyl diphosphate synthase (UPPs). The observed trend... |
|
|
Light conditions alter accumulation of long chain polyprenols in leaves of trees and shrubs throughout the vegetation season.
Acta Biochim. Pol. 52(1) , 233-41, (2005) In many plants belonging to angiosperms and gymnosperms the accumulation in leaves of long chain polyprenols and polyprenyl esters during growth in natural habitats depends on the light intensity. The amount of polyprenols in leaves is also positively correla... |