Ethyl 2-butynoate
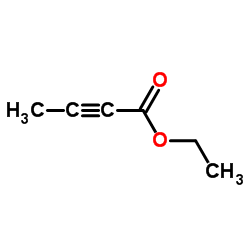
Ethyl 2-butynoate structure
|
Common Name | Ethyl 2-butynoate | ||
|---|---|---|---|---|
| CAS Number | 4341-76-8 | Molecular Weight | 112.127 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 163.0±9.0 °C at 760 mmHg | |
| Molecular Formula | C6H8O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 53.2±6.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Rhodium-catalyzed regio- and stereoselective codimerization of alkenes and electron-deficient internal alkynes leading to 1,3-dienes.
Org. Lett. 10(13) , 2829-31, (2008) A cationic rhodium(I)/H(8)-BINAP complex catalyzes codimerization of alkenes bearing no alpha-hydrogen and electron-deficient internal alkynes, leading to 1,3-dienes in good yields with moderate to excellent regio- and stereoselectivity. The same complex also... |
|
|
Phosphine-catalyzed annulation of thioamides and 2-alkynoates: a new synthesis of thiazolines.
J. Org. Chem. 67(13) , 4595-8, (2002) The annulation of thioamides with 2-alkynoates and 2,3-dienoates under the catalysis of tri-n-butylphosphine was described. The annulation reaction provided a new entry to thiazolines, particularly those with 2-aryl substituents. |
|
|
Increases in microbial nitrogen production and efficiency in vitro with three inhibitors of ruminal methanogenesis.
Can. J. Microbiol. 53(4) , 496-503, (2007) It was hypothesized that the addition of crotonic acid or 3-butenoic acid would relieve constraints in digestibility observed when methane formation is inhibited by lumazine, propynoic acid, or ethyl 2-butynoate. In six incubations, one of the three methanoge... |
|
|
Synthesis of 2-azaspiro [4.4] nonan-1-ones via phosphine-catalysed [3+ 2]-cycloadditions. Yong SR, et al.
Tetrahedron 61(34) , 8120-8129, (2005)
|
|
|
Dichloro [TADDOLato (2-)-O,O'] titanium/Dichlorobis [1-methylethoxy] titanium-Mediated, Highly Diastereo-and Enantioselective Additions of Silyl Enol Ethers to Nitro Olefins and [3+ 2] Cycloadditions of Primary Adducts to Acetylenes. Seebach D, et al.
Helv. Chim. Acta 82(11) , 1829-1842, (1999)
|
|
|
Efficient and stereoselective cross-coupling with highly substituted alkenylsilanols. Denmark SE and Pan W.
J. Organomet. Chem. 653(1) , 98-104, (2002)
|