Deflazacort
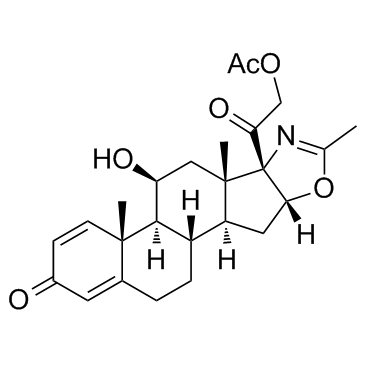
Deflazacort structure
|
Common Name | Deflazacort | ||
|---|---|---|---|---|
| CAS Number | 14484-47-0 | Molecular Weight | 441.517 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 595.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C25H31NO6 | Melting Point | 255-256.5ºC | |
| MSDS | Chinese USA | Flash Point | 313.9±30.1 °C | |
|
Health-related quality of life in children and adolescents with Duchenne muscular dystrophy.
Pediatrics 130(6) , e1559-66, (2012) The purpose of this study was to assess health-related quality of life (QoL) in children with Duchenne muscular dystrophy (DMD), including development and field-testing of a DMD-specific module integrated with the core Pediatric Quality of Life Inventory (Ped... |
|
|
Early corticosteroid treatment in 4 Duchenne muscular dystrophy patients: 14-year follow-up.
Muscle Nerve 45(6) , 796-802, (2012) Corticosteroid treatment is the standard of care in Duchenne muscular dystrophy (DMD), but the optimal age to initiate treatment and dosage pattern remain a matter of discussion.We performed a long-term study of alternate-day corticosteroids in five 2- to 4-y... |
|
|
Treatment of dysferlinopathy with deflazacort: a double-blind, placebo-controlled clinical trial.
Orphanet J. Rare Dis. 8 , 26, (2013) Dysferlinopathies are autosomal recessive disorders caused by mutations in the dysferlin (DYSF) gene encoding the dysferlin protein. DYSF mutations lead to a wide range of muscular phenotypes, with the most prominent being Miyoshi myopathy (MM) and limb girdl... |
|
|
Acute and chronic corticosteroid treatment of ten patients with paralytic form of Sydenham's chorea.
Eur. J. Paediatr. Neurol. 16(4) , 373-8, (2012) To determine efficacy and safety of corticosteroid treatment in patients with severe Sydenham's chorea paralytic form.This is a 4 years observational study on ten patient with severe paralytic form of Sydenham's chorea unresponsive to neuroleptics and antiepi... |
|
|
Oral pemphigus: long term behaviour and clinical response to treatment with deflazacort in sixteen cases.
J. Oral. Pathol. Med. 29(4) , 145-52, (2000) Systemic corticosteroids remain the mainstay of therapy for pemphigus. Their use has transformed what was almost invariably a fatal illness into one whose mortality is now below 10%. Unfortunately, the high doses and prolonged administration of corticosteroid... |
|
|
Growth hormone treatment in boys with Duchenne muscular dystrophy and glucocorticoid-induced growth failure.
Neuromuscul. Disord. 22(12) , 1046-56, (2012) This study evaluated efficacy and safety of growth hormone treatment in Duchenne muscular dystrophy boys with glucocorticoid-induced growth failure. We reviewed 39 consecutive boys (average age 11.5 years; 32 ambulatory) treated with growth hormone for 1 year... |
|
|
Novel approaches to corticosteroid treatment in Duchenne muscular dystrophy.
Phys. Med. Rehabil. Clin. N. Am. 23(4) , 821-8, (2012) Although prednisone has never been formally approved for use in Duchenne muscular dystrophy (DMD) by regulatory agencies, its efficacy has been confirmed in trials dating from the 1980s. There is a strong need for optimization of both specific type of glucoco... |
|
|
Siliconosis: autoimmune/inflammatory syndrome induced by adjuvants (ASIA).
Isr. Med. Assoc. J. 14(2) , 137-8, (2012)
|
|
|
Worsening of cardiomyopathy using deflazacort in an animal model rescued by gene therapy.
PLoS ONE 6(9) , e24729, (2011) We have previously demonstrated that gene therapy can rescue the phenotype and extend lifespan in the delta-sarcoglycan deficient cardiomyopathic hamster. In patients with similar genetic defects, steroids have been largely used to slow down disease progressi... |
|
|
Deflazacort: a glucocorticoid with few metabolic adverse effects but important immunosuppressive activity.
Adv. Ther. 24(5) , 1052-60, (2007) Deflazacort (DFZ) is a synthetic glucocorticoid that has few adverse effects on glucose and calcium metabolism and fewer deleterious effects on the neuronal population. Therefore, it may have a crucial role in the treatment of patients with autoimmune disorde... |