Novel approaches to corticosteroid treatment in Duchenne muscular dystrophy.
Eric P Hoffman, Erica Reeves, Jesse Damsker, Kanneboyina Nagaraju, John M McCall, Edward M Connor, Kate Bushby
Index: Phys. Med. Rehabil. Clin. N. Am. 23(4) , 821-8, (2012)
Full Text: HTML
Abstract
Although prednisone has never been formally approved for use in Duchenne muscular dystrophy (DMD) by regulatory agencies, its efficacy has been confirmed in trials dating from the 1980s. There is a strong need for optimization of both specific type of glucocorticoid (eg, prednisone, vs deflazacort or others) and the dosing regimen. Ideally an optimized regimen would maximize efficacy while minimizing side-effect profiles. A new trial, FOR-DMD, aims to address this gap in knowledge. In parallel, there has been progress in the area of "dissociative steroids," drugs that are able to better separate efficacy and side effects, providing a broader therapeutic window.Copyright © 2012 Elsevier Inc. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
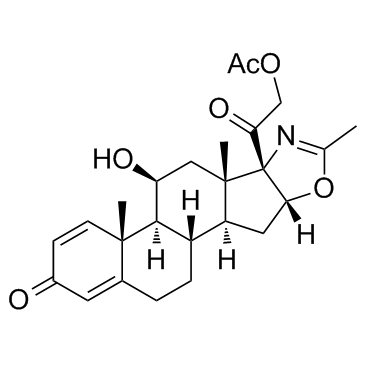 |
Deflazacort
CAS:14484-47-0 |
C25H31NO6 |
|
Health-related quality of life in children and adolescents w...
2012-12-01 [Pediatrics 130(6) , e1559-66, (2012)] |
|
Early corticosteroid treatment in 4 Duchenne muscular dystro...
2012-06-01 [Muscle Nerve 45(6) , 796-802, (2012)] |
|
Treatment of dysferlinopathy with deflazacort: a double-blin...
2013-01-01 [Orphanet J. Rare Dis. 8 , 26, (2013)] |
|
Acute and chronic corticosteroid treatment of ten patients w...
2012-07-01 [Eur. J. Paediatr. Neurol. 16(4) , 373-8, (2012)] |
|
Oral pemphigus: long term behaviour and clinical response to...
2000-04-01 [J. Oral. Pathol. Med. 29(4) , 145-52, (2000)] |