Bromochloromethane
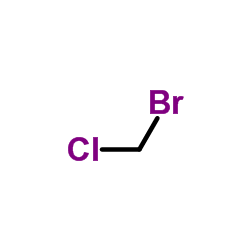
Bromochloromethane structure
|
Common Name | Bromochloromethane | ||
|---|---|---|---|---|
| CAS Number | 74-97-5 | Molecular Weight | 129.384 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 65.1±8.0 °C at 760 mmHg | |
| Molecular Formula | CH2BrCl | Melting Point | −88 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | -22.9±8.5 °C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
|
Predicting vehicle effects on the dermal absorption of halogenated methanes using physiologically based modeling.
Toxicol. Sci. 48(2) , 180-8, (1999) Occupational and environmental settings present opportunities for humans to come into contact with a variety of chemicals via the dermal route. The chemicals contacting the skin are likely to be diluted with a vehicle or present as a component of a mixture. I... |
|
|
Effect of bromochloromethane on methane emission, rumen fermentation pattern, milk yield, and fatty acid profile in lactating dairy goats.
J. Dairy Sci. 95(4) , 2027-36, (2012) Several technologies have been tested to reduce enteric methanogenesis, but very few have been successfully used in practical conditions for livestock. Furthermore, the consequences of reduced rumen methane production on animal performance and milk quality ar... |
|
|
Effect of isoniazid or phenobarbital pretreatment on the metabolism of dihalomethanes to carbon monoxide.
Pol. J. Occup. Med. Environ. Health 5(3) , 245-50, (1992) An oral dose of 6.2 mmoles of diachloromethane (DCM), bromochloromethane (BCM) or dibromomethane (DBM) per kg body mass yielded a maximum carboxyhemoglobin (COHb) level of about 9% (at 6 hr), 11% (at 8 hr) and 22% (at 12 hr), respectively. Pretreatment of rat... |
|
|
Expression of mammalian glutathione S-transferase 5-5 in Salmonella typhimurium TA1535 leads to base-pair mutations upon exposure to dihalomethanes.
Proc. Natl. Acad. Sci. U. S. A. 90(18) , 8576-80, (1993) Dihalomethanes can produce liver tumors in mice but not in rats, and concern exists about the risk of these compounds to humans. Glutathione (GSH) conjugation of dihalomethanes has been considered to be a critical event in the bioactivation process, and risk ... |
|
|
Inhibition of methanogens by bromochloromethane: effects on microbial communities and rumen fermentation using batch and continuous fermentations.
Br. J. Nutr. 101(10) , 1484-92, (2009) Bromochloromethane (BCM), a halogenated methane analogue, was evaluated for its anti-methanogenic activity in both batch and continuous fermentation systems. For batch fermentation, a roughage-based substrate was incubated with mixed rumen microflora for 24 h... |
|
|
A physiologically based simulation approach for determining metabolic constants from gas uptake data.
Toxicol. Appl. Pharmacol. 86(3) , 341-52, (1986) In vivo metabolic constants were determined in male Fischer rats for five chemicals: 1,1-dichloroethylene (1,1-DCE), diethyl ether (DE), bromochloromethane (BCM), methyl chloroform (MC), and carbon tetrachloride (CCl4). A closed recirculated exposure system w... |
|
|
A physiological pharmacokinetic model for dermal absorption of vapors in the rat.
Toxicol. Appl. Pharmacol. 85(2) , 286-94, (1986) Absorption of chemical vapors through the skin is a passive process that is not easily quantitated, but may be important in the assessment of health hazards in some occupational circumstances. Physiological modeling is a quantitative technique which may provi... |
|
|
Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane.
FEMS Microbiol. Ecol. 62(3) , 313-22, (2007) Methyl coenzyme-M reductase A (mcrA) clone libraries were generated from microbial DNA extracted from the rumen of cattle fed a roughage diet with and without supplementation of the antimethanogenic compound bromochloromethane. Bromochloromethane reduced tota... |
|
|
Determining kinetic constants of chlorinated ethane metabolism in the rat from rates of exhalation.
Toxicol. Appl. Pharmacol. 99(2) , 344-53, (1989) The kinetic constants of chemical metabolism are used to develop physiologically based pharmacokinetic (PB-PK) models which predict the time course distribution of volatile chemicals in mammalian systems. Gas uptake techniques have proved useful in determinin... |
|
|
Physiologically based modeling of nonsteady state dermal absorption of halogenated methanes from an aqueous solution.
Toxicol. Appl. Pharmacol. 144(2) , 315-24, (1997) Dermal absorption of organic chemicals from aqueous solutions are a concern in both the workplace and the home. Organic chemicals are generally not very soluble in water and the exposure may never reach steady state because the concentration of chemical decre... |