Zolmitriptan
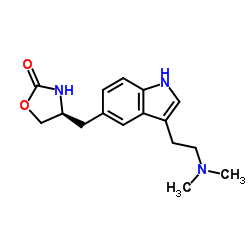
Zolmitriptan structure
|
Common Name | Zolmitriptan | ||
|---|---|---|---|---|
| CAS Number | 139264-17-8 | Molecular Weight | 287.357 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 563.3±38.0 °C at 760 mmHg | |
| Molecular Formula | C16H21N3O2 | Melting Point | 136-141ºC | |
| MSDS | Chinese USA | Flash Point | 294.5±26.8 °C | |
|
Bioenhanced sublingual tablet of drug with limited permeability using novel surfactant binder and microencapsulated polysorbate: In vitro/in vivo evaluation.
Eur. J. Pharm. Biopharm. 94 , 386-92, (2015) Formulation of sublingual tablets of drugs with limited permeability poses a great challenge due to their poor absorption. In this study, bioenhanced sublingual tablets (BESTs) of zolmitriptan were prepared using novel surfactant binder (Pluronic® p123/Syloid... |
|
|
Zolmitriptan: a novel portal hypotensive agent which synergizes with propranolol in lowering portal pressure.
PLoS ONE 8(1) , e52683, (2013) Only a limited proportion of patients needing pharmacological control of portal hypertension are hemodynamic responders to propranolol. Here we analyzed the effects of zolmitriptan on portal pressure and its potential interaction with propranolol.ZOLMITRIPTAN... |
|
|
Preparation and evaluation of zolmitriptan submicron emulsion for rapid and effective nasal absorption in beagle dogs.
Drug Dev. Ind. Pharm. 37(12) , 1509-16, (2011) Submicron emulsion was prepared for rapid and effective nasal absorption of zolmitriptan (ZT). The different charge inducers and pH values of the formulations were evaluated to optimize the formulations. Submicron emulsion prepared by using stearylamine as po... |
|
|
Evaluation of submicron emulsion as vehicles for rapid-onset intranasal delivery and improvement in brain targeting of zolmitriptan.
Drug Deliv. 18(8) , 578-85, (2011) This study was to evaluate submicron emulsion as a drug carrier for intranasal delivery of zolmitriptan (ZT). Since the drug distribution in submicron emulsion might influence the nasal absorption, two different formulations separately incorporating the drug ... |
|
|
Quantitative determination of zolmitriptan in rat blood and cerebrospinal fluid by reversed phase HPLC-ESI-MS/MS analysis: application to in vivo preclinical pharmacokinetic study.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 901 , 72-8, (2012) A fast HPLC-ESI-MS/MS method has been developed and validated for the quantification of the potent and selective antimigraine zolmitriptan in rat blood and cerebrospinal fluid (CSF). The assay has been then applied for in vivo preclinical studies. The analyti... |
|
|
Intranasal zolmitriptan for the treatment of acute migraine.
Headache 53 Suppl 2 , 62-71, (2013) To review the pharmacokinetics, efficacy, tolerability, and patient acceptance of zolmitriptan nasal spray (NS).Gastroparesis may delay or diminish the absorption of oral triptans, and nausea or vomiting may do the same and/or make it difficult to take a tabl... |
|
|
Determination of zolmitriptan and its primary metabolite, n-desmethy-zolmitriptan, in rat plasma by LC-MS-MS.
J. Chromatogr. Sci. 51(1) , 59-64, (2013) The objective of this work was to develop a simple, cost effective, rugged and rapid method for the simultaneous estimation of zolmitriptan (ZP) and its active metabolite n-desmethy-zolmitriptan (DZP) in rat plasma to meet the requirement for biological sampl... |
|
|
Part III: the convenience of, and patient preference for, zolmitriptan orally disintegrating tablet.
Curr. Med. Res. Opin. 21 Suppl 3 , S13-7, (2005) As part of an optimal strategy for the management of migraine, the individual needs and preferences of patients need to be considered when of patients need to be considered when prescribing treatments. Zolmitriptan has been available as a conventional oral ta... |
|
|
Reassessing the contraindication of zolmitriptan and serotonin reuptake inhibitors: an evidence-based pharmacotherapeutic case report.
J. Clin. Pharm. Ther. 28(1) , 69-72, (2003)
|
|
|
Fast relief from migraine attacks using fast-disintegrating sublingual zolmitriptan tablets.
Drug Dev. Ind. Pharm. 38(6) , 762-9, (2012) Zolmitriptan is a potent molecule for treatment of migraine. Its current oral therapies present drawbacks such as slow onset of action, low bioavailability and large inter-subject variability. Fast disintegrating sublingual zolmitriptan tablet (FDST) using fr... |