Part III: the convenience of, and patient preference for, zolmitriptan orally disintegrating tablet.
Andrew J Dowson, Per Almqvist
Index: Curr. Med. Res. Opin. 21 Suppl 3 , S13-7, (2005)
Full Text: HTML
Abstract
As part of an optimal strategy for the management of migraine, the individual needs and preferences of patients need to be considered when of patients need to be considered when prescribing treatments. Zolmitriptan has been available as a conventional oral tablet for more than seven years, and is established as a highly effective, well-tolerated compound for the acute treatment of migraine. A bioequivalent, orally disintegrating tablet (ODT) of zolmitriptan, which dissolves on the tongue without the need for additional fluid intake, has been developed. In a study designed to compare patient preference for zolmitriptan ODT and conventional oral sumatriptan tablets, > 60% of the 186 patients questioned had an overall preference for zolmitriptan ODT, with > 80% of patients reporting that this was the more convenient and less disruptive therapy to take. Approximately 90% of patients agreed that, unlike a conventional tablet, zolmitriptan ODT can be taken wherever and whenever a migraine occurs. When patient preference for zolmitriptan ODT and the ODT formulation of rizatriptan was compared in 171 migraineurs, 70% had an overall preference for zolmitriptan ODT to be superior to rizatriptan ODT with respect to taste and aftertaste, as well as packaging. In summary, not only is zolmitriptan ODT a convenient tablets, such as the sumatriptan oral tablet, but patients generally consider it to be a more attractive option for the acute treatment of migraine than the orally disintegrating version of rizatriptan.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
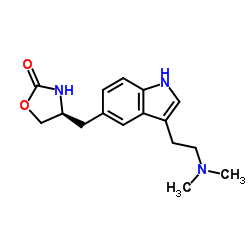 |
Zolmitriptan
CAS:139264-17-8 |
C16H21N3O2 |
|
Bioenhanced sublingual tablet of drug with limited permeabil...
2015-08-01 [Eur. J. Pharm. Biopharm. 94 , 386-92, (2015)] |
|
Zolmitriptan: a novel portal hypotensive agent which synergi...
2013-01-01 [PLoS ONE 8(1) , e52683, (2013)] |
|
Preparation and evaluation of zolmitriptan submicron emulsio...
2011-12-01 [Drug Dev. Ind. Pharm. 37(12) , 1509-16, (2011)] |
|
Evaluation of submicron emulsion as vehicles for rapid-onset...
2011-11-01 [Drug Deliv. 18(8) , 578-85, (2011)] |
|
Quantitative determination of zolmitriptan in rat blood and ...
2012-07-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 901 , 72-8, (2012)] |