6-Aminoquinoline
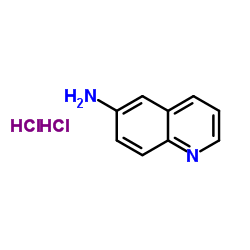
6-Aminoquinoline structure
|
Common Name | 6-Aminoquinoline | ||
|---|---|---|---|---|
| CAS Number | 580-15-4 | Molecular Weight | 144.17 | |
| Density | N/A | Boiling Point | 192 °C | |
| Molecular Formula | C9H8N2 | Melting Point | 115-119 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 146°C/0.3mm | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
A quantitative metabolomics peek into planarian regeneration.
Analyst 140 , 3445-64, (2015) The fresh water planarian species Schmidtea mediterranea is an emerging stem cell model because of its capability to regenerate a whole animal from a small piece of tissue. It is one of the best model systems to address the basic mechanisms essential for rege... |
|
|
Aminoquinolines as fluorescent labels for hydrophilic interaction liquid chromatography of oligosaccharides.
Biol. Chem. 393(8) , 757-65, (2012) In this study, we investigated the potential of four different aminoquinoline (AQ) compounds as fluorescent labels for glycan analysis using hydrophilic interaction liquid chromatography (HILIC) and fluorescence detection (FLD). We confirmed the optimal excit... |
|
|
From 6-aminoquinolone antibacterials to 6-amino-7-thiopyranopyridinylquinolone ethyl esters as inhibitors of Staphylococcus aureus multidrug efflux pumps.
J. Med. Chem. 53 , 4466-80, (2010) The thiopyranopyridine moiety was synthesized as a new heterocyclic base to be inserted at the C-7 position of selected quinolone nuclei followed by a determination of antibacterial activity against strains of Staphylococcus aureus. Selected thiopyranopyridin... |
|
|
The 6-aminoquinolone WC5 inhibits human cytomegalovirus replication at an early stage by interfering with the transactivating activity of viral immediate-early 2 protein.
Antimicrob. Agents Chemother. 54 , 1930-40, (2010) WC5 is a 6-aminoquinolone that potently inhibits the replication of human cytomegalovirus (HCMV) but has no activity, or significantly less activity, against other herpesviruses. Here we investigated the nature of its specific anti-HCMV activity. Structure-ac... |
|
|
Simultaneous determination of nicotine and cotinine in serum using high-performance liquid chromatography with fluorometric detection and postcolumn UV-photoirradiation system.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 934 , 41-5, (2013) A simple and rapid method for the simultaneous determination of serum nicotine and cotinine using high-performance liquid chromatography (HPLC)-fluorometric detection with a postcolumn ultraviolet-photoirradiation system was developed. Analytes were extracted... |
|
|
Application of the dipeptidyl peptidase IV (DPPIV/CD26) based prodrug approach to different amine-containing drugs.
J. Med. Chem. 53 , 559-72, (2010) Here we explore the applicability of the dipeptidyl peptidase IV (DPPIV/CD26) based prodrug approach to a variety of amine-containing drugs. Efficient procedures have been developed for the synthesis of dipeptide and tetrapeptide amide prodrugs including N-ac... |
|
|
A fluorogenic, small molecule reporter for mammalian phospholipase C isozymes.
ACS Chem. Biol. 6(3) , 223-8, (2011) Phospholipase C isozymes (PLCs) catalyze the conversion of the membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP(2)) into two second messengers, inositol 1,4,5-trisphosphate and diacylglycerol. This family of enzymes are key signaling proteins that re... |
|
|
6-aminoquinoline as a fluorogenic leaving group in peptide cleavage reactions: a new fluorogenic substrate for chymotrypsin.
Anal. Biochem. 116(2) , 408-13, (1981)
|
|
|
trans-Stilbene oxide induces expression of genes involved in metabolism and transport in mouse liver via CAR and Nrf2 transcription factors.
Anal. Biochem. 126(2) , 447-55, (1982) trans-Stilbene oxide (TSO) induces drug metabolizing enzymes in rat and mouse liver. TSO is considered a phenobarbital-like compound because it induces Cyp2B mRNA expression in liver. Phenobarbital increases Cyp2B expression in liver via activation of the con... |
|
|
Capillary zone electrophoresis of linear and branched oligosaccharides.
J. Chromatogr. A. 600 , 279, (1992) The electrophoretic behavior of derivatized linear and branched oligosaccharides from various sources was examined in capillary zone electrophoresis with polyether-coated fused-silica capillaries. Two UV-absorbing (also fluorescent) derivatizing agents (2-ami... |