2-(4-Hydroxyphenylazo)benzoic acid
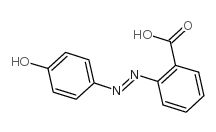
2-(4-Hydroxyphenylazo)benzoic acid structure
|
Common Name | 2-(4-Hydroxyphenylazo)benzoic acid | ||
|---|---|---|---|---|
| CAS Number | 1634-82-8 | Molecular Weight | 242.23000 | |
| Density | 1.3g/cm3 | Boiling Point | 493.995ºC at 760 mmHg | |
| Molecular Formula | C13H10N2O3 | Melting Point | 204-208 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 252.559ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Development of an efficient signal amplification strategy for label-free enzyme immunoassay using two site-specific biotinylated recombinant proteins.
Anal. Chim. Acta 859 , 66-71, (2015) Constructing a recombinant protein between a reporter enzyme and a detector protein to produce a homogeneous immunological reagent is advantageous over random chemical conjugation. However, the approach hardly recombines multiple enzymes in a difunctional fus... |
|
|
Synthesis and characterization of alginate/poly-L-ornithine/alginate microcapsules for local immunosuppression.
J. Microencapsul. 283 , 840-848, (2008) Alginate/poly-L-ornithine/alginate (APA) coherent microencapsulation, which provides an immunoselective and highly biocompatible membrane, creates a viable option for cellular or tissue transplantation. This study explored the potential of incorporating immun... |
|
|
Biotinylated thermoresponsive core cross-linked nanoparticles via RAFT polymerization and "click" chemistry.
J. Colloid. Interface Sci. 356(1) , 16-23, (2011) A straightforward approach to the synthesis of "clickable" thermoresponsive core cross-linked (CCL) nanoparticles was developed. This approach was based on reversible addition-fragmentation chain transfer (RAFT) radical cross-linking polymerization of styrene... |
|
|
Binding properties of HABA-type azo derivatives to avidin and avidin-related protein 4.
Chem. Biol. 13(10) , 1029-39, (2006) The chicken genome encodes several biotin-binding proteins, including avidin and avidin-related protein 4 (AVR4). In addition to D-biotin, avidin binds an azo dye compound, 4-hydroxyazobenzene-2-carboxylic acid (HABA), but the HABA-binding properties of AVR4 ... |
|
|
Rational modification of ligand-binding preference of avidin by circular permutation and mutagenesis.
ChemBioChem. 9(7) , 1124-35, (2008) Chicken avidin is a key component used in a wide variety of biotechnological applications. Here we present a circularly permuted avidin (cpAvd4-->3) that lacks the loop between beta-strands 3 and 4. Importantly, the deletion of the loop has a positive effect ... |
|
|
Determination of structural, spectrometric and nonlinear optical features of 2-(4-hydroxyphenylazo)benzoic acid by experimental techniques and quantum chemical calculations.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 105 , 80-7, (2013) The optimized geometrical structure, vibrational and electronic transitions, chemical shifts and nonlinear optical properties of 2-(4-hydroxyphenylazo)benzoic acid (HABA) compound were presented in this study. The ground state geometrical structure and vibrat... |
|
|
Adhesion from tethered ligand-receptor bonds with microsecond lifetimes.
Langmuir 24(4) , 1212-8, (2008) According to classical thermodynamics, biological ligand-receptor bonds should have a median lifetime of about 2 ms, and nearly half should have lifetimes of nanoseconds to microseconds. As a result, it is clear that many "weak" bonds are indispensable for ce... |
|
|
A labeling, detection, and purification system based on 4-hydroxyazobenzene-2-carboxylic acid: an extension of the avidin-biotin system.
Anal. Biochem. 284(2) , 354-66, (2000) We introduce a new nonradioactive, chromogenic label based on 4-hydroxyazobenzene-2-carboxylic acid (HABA), which is suitable for bioanalytical application, e.g., detection, localization, isolation, and purification. The HABA label is superior to other system... |
|
|
Highly selective cyclic peptide ligands for NeutrAvidin and avidin identified by phage display.
Chem. Biol. Drug Des. 68(1) , 3-10, (2006) Screening combinatorial libraries of conformationally constrained peptides against macromolecular targets is utilized in identifying novel drug leads and in developing new reagents for chemical biology. In methods such as phage-display selections, biotinylate... |
|
|
Branching of o-nitrobenzoate degradation pathway in Arthrobacter protophormiae RKJ100: identification of new intermediates.
FEMS Microbiol. Lett. 229(2) , 231-6, (2003) We have earlier reported a novel reductive pathway for o-nitrobenzoate (ONB) degradation (at 0.5 mM) in Arthrobacter protophormiae RKJ100, which proceeds via the formation of o-hydroxylaminobenzoate (HABA) and anthranilate (AA). During growth of this organism... |