Vanadium(V) trichloride oxide
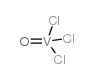
Vanadium(V) trichloride oxide structure
|
Common Name | Vanadium(V) trichloride oxide | ||
|---|---|---|---|---|
| CAS Number | 7727-18-6 | Molecular Weight | 173.30000 | |
| Density | 1.84 g/mL at 25 °C(lit.) | Boiling Point | 126-127 °C(lit.) | |
| Molecular Formula | Cl3OV | Melting Point | −77 °C(lit.) | |
| MSDS | N/A | Flash Point | 126-127°C | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
|
[Hygienic characteristics of the working conditions in the manufacture of vanadium hydroxychloride].
Gig. Tr. Prof. Zabol. (1) , 51-2, (1985)
|
|
|
The gas-phase on-line production of vanadium oxytrihalides, VOX3 and their identification by infrared spectroscopy.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 56(14) , 2693-8, (2000) A new route has been devised, leading to the production of VOX3 molecules where X=F, Br and I by an on-line process using vanadium oxytrichloride, VOCl3 as a starting compound passed over the following heated salts NaF, KBr and KI at 375, 700 and 550 degrees ... |
|
|
Aerobic oxidation of thiols to disulfides catalyzed by trichlorooxyvanadium.
Chem. Pharm. Bull. 52(5) , 625-7, (2004) Thiols were converted into disulfide by the aerobic oxidation catalyzed by trichlorooxyvanadium in the presence of molecular sieves 3A. |
Journals:
More...