S-(+)-FLUOXETINE HYDROCHLORIDE
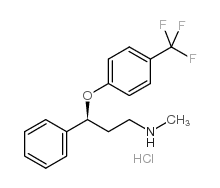
S-(+)-FLUOXETINE HYDROCHLORIDE structure
|
Common Name | S-(+)-FLUOXETINE HYDROCHLORIDE | ||
|---|---|---|---|---|
| CAS Number | 114247-06-2 | Molecular Weight | 345.78700 | |
| Density | N/A | Boiling Point | 395.1ºC at 760 mmHg | |
| Molecular Formula | C17H19ClF3NO | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 192.8ºC | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum.
Nat. Chem. Biol. 5 , 765-71, (2009) Studies of gene function and molecular mechanisms in Plasmodium falciparum are hampered by difficulties in characterizing and measuring phenotypic differences between individual parasites. We screened seven parasite lines for differences in responses to 1,279... |
|
|
Case history: the discovery of fluoxetine hydrochloride (Prozac).
Nat. Rev. Drug Discov. 4(9) , 764-74, (2005) In the early 1970s, evidence of the role of serotonin (5-hydroxytryptamine or 5-HT) in depression began to emerge and the hypothesis that enhancing 5-HT neurotransmission would be a viable mechanism to mediate antidepressant response was put forward. On the b... |
|
|
Microautoradiography of [123I]ADAM in mice treated with fluoxetine and serotonin reuptake inhibitors.
Nucl. Med. Biol. 31(5) , 557-62, (2004) A radiopharmaceutical, (123)I-labeled 2-((2-((dimethylamino)methyl)phenyl)thio)-5-iodophenylamine ([(123)I]ADAM), has been developed recently for evaluation of how serotonin transporters (SERT) function in the brain. However, the detailed biodistribution and ... |