n-methylbenzol-1,2-diamin
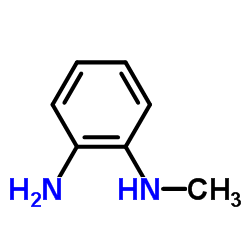
n-methylbenzol-1,2-diamin structure
|
Common Name | n-methylbenzol-1,2-diamin | ||
|---|---|---|---|---|
| CAS Number | 4760-34-3 | Molecular Weight | 122.168 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 252.4±23.0 °C at 760 mmHg | |
| Molecular Formula | C7H10N2 | Melting Point | 22 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 122.4±26.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Modification of carbon electrode with aryl groups having an aliphatic amine by electrochemical reduction of in situ generated diazonium cations.
Langmuir 24(16) , 8711-8, (2008) The electrochemically induced functionalization of glassy carbon electrode by aryl groups having an aliphatic amine group was achieved by reduction of in situ generated diazonium cations in aqueous media. The corresponding diazonium cations of 4-aminobenzylam... |
|
|
1-Methyl-1H-benzimidazole-2(3H)-thione.
Acta Crystallogr. Sect. E Struct. Rep. Online 64(Pt 6) , o1141, (2008) The title compound, C(8)H(8)N(2)S, was prepared by the condensation of N-methyl-1,2-phenyl-enediamine and carbon disulfide. The crystal structure is stabilized by a C-H⋯π inter-action between a benzene H atom and the benzene ring of a neighbouring mol-ecule, ... |
|
|
Efficient and improved synthesis of Telmisartan.
Beilstein J. Org. Chem. 6 , 25, (2010) An efficient synthesis of the angiotensin II receptor antagonist Telmisartan (1) is presented involving a cross coupling of 4-formylphenylboronic acid 10 with 2-(2-bromophenyl)-4,4-dimethyl-2-oxazoline (11) as the key step (90% yield). The benzimidazole moiet... |
|
|
Synthesis , 1273, (1992)
|
|
|
Recent developments in use of heteropolyacids, their salts and polyoxometalates in organic synthesis. Heravi MM and Sadjadi S.
J. Iranian Chem. Soc. 6(1) , 1-54, (2009)
|