Acridone
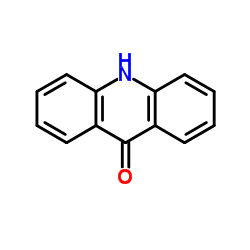
Acridone structure
|
Common Name | Acridone | ||
|---|---|---|---|---|
| CAS Number | 578-95-0 | Molecular Weight | 195.217 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 355.0±12.0 °C at 760 mmHg | |
| Molecular Formula | C13H9NO | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 155.0±19.7 °C | |
|
Copper-catalyzed intramolecular oxidative C-H functionalization and C-N formation of 2-aminobenzophenones: unusual pseudo-1,2-shift of the substituent on the aryl ring.
Chemistry 19(2) , 460-4, (2013)
|
|
|
Online Investigation of Aqueous-Phase Electrochemical Reactions by Desorption Electrospray Ionization Mass Spectrometry.
J. Am. Soc. Mass Spectrom. 26 , 1676-85, (2015) Electrochemistry (EC) combined with mass spectrometry (MS) is a powerful tool for elucidation of electrochemical reaction mechanisms. However, direct online analysis of electrochemical reaction in aqueous phase was rarely explored. This paper presents the onl... |
|
|
Extravascular transport of drugs in tumor tissue: effect of lipophilicity on diffusion of tirapazamine analogues in multicellular layer cultures.
J. Med. Chem. 48 , 1079-87, (2005) The extravascular diffusion of antitumor agents is a key determinant of their therapeutic activity, but the relationships between physicochemical properties of drugs and their extravascular transport are poorly understood. It is well-known that drug lipophili... |
|
|
Structure-activity relationship studies of acridones as potential antipsoriatic agents. 1. Synthesis and antiproliferative activity of simple N-unsubstituted 10H-acridin-9-ones against human keratinocyte growth.
Eur. J. Med. Chem. 45 , 3299-310, (2010) A series of N-unsubstituted hydroxy-10H-acridin-9-ones were synthesized and evaluated for inhibitory action against HaCaT keratinocyte growth, in order to explore their potential as antipsoriatic agents. For structure-activity relationship studies, the number... |
|
|
Acridone-labeled DNA aptamer for the detection of biomolecules.
Nucleic Acids Symp. Ser. (53) , 255-6, (2009) An acridone-labeled DNA aptamer was synthesized to produce an aptamer probe. This aptamer probe could detect a specific molecule based on conformational changes upon binding of the specific molecules and the quenching of acridone emission. |
|
|
Presence and ex vivo formation of acridone in blood of patients routinely treated with carbamazepine: exploration of the 9-acridinecarboxaldehyde pathway.
Xenobiotica 41(2) , 91-100, (2011) Carbamazepine (CBZ) is a useful anticonvulsive drug associated with rare severe adverse drug reactions. The physio-pathological mechanisms of these reactions are unknown although evidence of immunological activation has been reported. The ability of 9-acridin... |
|
|
The acridone derivative MBLI-87 sensitizes breast cancer resistance protein-expressing xenografts to irinotecan.
Eur. J. Cancer 47(4) , 640-8, (2011) The breast cancer resistance protein ABCG2 confers cellular resistance to irinotecan (CPT-11) and its active metabolite SN-38. We utilised ABCG2-expressing xenografts as a model to evaluate the ability of a non-toxic ABCG2 inhibitor to increase intracellular ... |
|
|
Synthesis of o-(dimethylamino)aryl ketones, acridones, acridinium salts, and 1H-indazoles by the reaction of hydrazones and arynes.
J. Org. Chem. 77(24) , 11232-56, (2012) A novel, efficient route to biologically and pharmaceutically important o-(dimethylamino)aryl ketones, acridones, acridinium salts, and 1H-indazoles has been developed starting from readily available hydrazones of aldehydes and o-(trimethylsilyl)aryl triflate... |
|
|
Anti-leishmanial activity of plant-derived acridones, flavaglines, and sulfur-containing amides.
Vector Borne Zoonotic Dis. 11(7) , 793-8, (2011) Visceral and cutaneous leishmaniases are an important public health problem in endemic geographic regions in 88 countries worldwide, with around 12 million infected people. Treatment options are limited due to toxicity and teratogenicity of the available drug... |
|
|
ATP selective acridone based fluorescent probes for monitoring of metabolic events.
Chem. Commun. (Camb.) 47(15) , 4472-4, (2011) Acridones carrying an appropriate substituent at N-10 showed significant fluorescence changes on interacting with ATP in HEPES buffer at pH 7.2. The selectivity and sufficient binding of these probes with ATP could be useful for monitoring of metabolic proces... |