Butyraldehyde
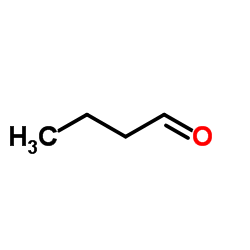
Butyraldehyde structure
|
Common Name | Butyraldehyde | ||
|---|---|---|---|---|
| CAS Number | 123-72-8 | Molecular Weight | 72.106 | |
| Density | 0.8±0.1 g/cm3 | Boiling Point | 77.6±3.0 °C at 760 mmHg | |
| Molecular Formula | C4H8O | Melting Point | -96 °C | |
| MSDS | Chinese USA | Flash Point | -11.1±0.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
|
Mosquito odorant receptor for DEET and methyl jasmonate.
Proc. Natl. Acad. Sci. U. S. A. 111(46) , 16592-7, (2014) Insect repellents are important prophylactic tools for travelers and populations living in endemic areas of malaria, dengue, encephalitis, and other vector-borne diseases. DEET (N,N-diethyl-3-methylbenzamide) is a 6-decade-old synthetic repellent, which is st... |
|
|
Comparison of Aroma-Active Volatiles in Oolong Tea Infusions Using GC-Olfactometry, GC-FPD, and GC-MS.
J. Agric. Food Chem. 63 , 7499-510, (2015) The aroma profile of oolong tea infusions (Dongdingwulong, DDWL; Tieguanyin, TGY; Dahongpao, DHP) were investigated in this study. Gas chromatography-olfactometry (GC-O) with the method of aroma intensity (AI) was employed to investigate the aroma-active comp... |
|
|
A novel ultrasound-assisted back extraction reverse micelles method coupled with gas chromatography-flame ionization detection for determination of aldehydes in heated edibles oils.
Food Chem. 188 , 30-6, (2015) A novel ultrasound-assisted back extraction reverse micelles coupled with gas chromatography-flame ionization detection has been developed for the extraction and determination of some short chain aldehydes in different heated edible oil samples. After the hom... |
|
|
OH-initiated photooxidations of 1-pentene and 2-methyl-2-propen-1-ol: mechanism and yields of the primary carbonyl products.
ChemPhysChem 15(17) , 3848-54, (2014) The products of the gas-phase reactions of OH radicals with 1-pentene and 2-methyl-2-propen-1-ol (221MPO) at T=298±2 K and atmospheric pressure were investigated by using a 4500 L atmospheric simulation chamber that was built especially for this work. The mol... |
|
|
Analysis of aldehydes in human exhaled breath condensates by in-tube SPME-HPLC.
Anal. Chim. Acta 900 , 67-75, (2015) In this paper, polypyrrole/graphene (PPy/G) composite coating was prepared by a facile electrochemical polymerization strategy on the inner surface of a stainless steel (SS) tube. Based on the coating tube, a novel online in-tube solid-phase microextraction -... |
|
|
Extension of a dynamic headspace multi-volatile method to milliliter injection volumes with full sample evaporation: Application to green tea.
J. Chromatogr. A. 1421 , 103-13, (2015) An extension of multi-volatile method (MVM) technology using the combination of a standard dynamic headspace (DHS) configuration, and a modified DHS configuration incorporating an additional vacuum module, was developed for milliliter injection volume of aque... |
|
|
Rapid analysis of trace volatile formaldehyde in aquatic products by derivatization reaction-based surface enhanced Raman spectroscopy.
Analyst 139(14) , 3614-21, (2014) Toxic formaldehyde is sometimes used illegally as a food preservative, however, on-site rapid analysis of trace formaldehyde in aquatic products remains a challenge. In this work, a simple on-site rapid quantification method for trace volatile formaldehyde in... |
|
|
The role of hydroxyl group acidity on the activity of silica-supported secondary amines for the self-condensation of n-butanal.
ChemSusChem 8(3) , 466-72, (2015) The catalytic activity of secondary amines supported on mesoporous silica for the self-condensation of n-butanal to 2-ethylhexenal can be altered significantly by controlling the Brønsted acidity of M--OH species present on the surface of the support. In this... |
|
|
Statistical thermodynamics of 1-butanol, 2-methyl-1-propanol, and butanal.
J. Chem. Phys. 136(3) , 034306, (2012) The purpose of the present investigation is to calculate partition functions and thermodynamic quantities, viz., entropy, enthalpy, heat capacity, and Gibbs free energies, for 1-butanol, 2-methyl-1-propanol, and butanal in the vapor phase. We employed the mul... |
|
|
Thermochemistry of radicals formed by hydrogen abstraction from 1-butanol, 2-methyl-1-propanol, and butanal.
J. Chem. Phys. 137(10) , 104314, (2012) We calculate the standard state entropy, heat capacity, enthalpy, and Gibbs free energy for 13 radicals important for the combustion chemistry of biofuels. These thermochemical quantities are calculated from recently proposed methods for calculating partition... |