6-aminocarbovir
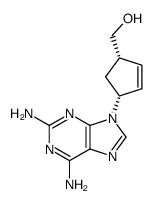
6-aminocarbovir structure
|
Common Name | 6-aminocarbovir | ||
|---|---|---|---|---|
| CAS Number | 124752-25-6 | Molecular Weight | 246.26800 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C11H14N6O | Melting Point | 180-182°C | |
| MSDS | N/A | Flash Point | N/A | |
| Symbol |

GHS05 |
Signal Word | Danger | |
|
Comparative brain exposure to (-)-carbovir after (-)-carbovir or (-)-6-aminocarbovir intravenous infusion in rats.
Pharm. Res. 12(6) , 911-5, (1995) Evaluate the ability of (-)-6-aminocarbovir ((-)-6AC) to improve the CNS exposure to (-)-carbovir ((-)-CBV).Activation of (-)-6AC in vitro was assessed by incubations of rat brain tissue homogenates. The in vivo brain exposure to (-)-CBV was then examined in ... |
|
|
Pharmacokinetic evaluation of (-)-6-aminocarbovir as a prodrug for (-)-carbovir in rats.
Drug Metab. Dispos. 20(1) , 47-51, (1992) The recently synthesized carbocyclic 2',3'-didehydro-2',3'-dideoxy-6-deoxy-6-amino-guanosine [(-)6AC] was evaluated as a prodrug for carbovir, carbocyclic 2',3'-didehydro-2',3'-dideoxyguanosine [(-)CBV] in seven male Sprague-Dawley rats. A randomized three-wa... |
|
|
First-pass disposition of (-)-6-aminocarbovir in rats. I. Prodrug activation may be limited by access to enzyme.
Drug Metab. Dispos. 27(1) , 113-21, (1999) Several in vitro and in situ approaches were used to determine the dominant presystemic activation site for (-)-6-aminocarbovir, (-)-carbocyclic 2',3'-didehydro-2', 3'-dideoxy-6-deoxy-6-aminoguanosine (6AC) conversion in rats. The in vitro disappearance half-... |
|
|
Evaluation of gastrointestinal absorption and metabolism.
Drug Metab. Rev. 29(4) , 957-75, (1997)
|
|
|
First-pass disposition of (-)-6-aminocarbovir in rats: II. Inhibition of intestinal first-pass metabolism.
Drug Metab. Dispos. 28(6) , 672-9, (2000) A CBV [(-)-carbovir, (-)-carbocyclic 2',3'-didehydro-2', 3'-dideoxyguanosine] prodrug, 6AC [(-)-6-aminocarbovir, (-)-carbocyclic 2',3'-didehydro-2', 3'-dideoxy-6-deoxy-6-aminoguanosine], was previously evaluated in rats, and it exhibited superiority to the pa... |
|
|
(-)-6-Aminocarbovir pharmacokinetics and relative carbovir exposure in rats.
Drug Metab. Dispos. 19(2) , 462-6, (1991) The potential for the metabolic conversion of (-)-6-aminocarbovir to (-)-carbovir, a potent reverse transcriptase inhibitor effective against human immunodeficiency virus, has been examined in male Sprague-Dawley rats. Plasma (-)-6-aminocarbovir concentration... |
|
|
Lack of effect of catheterization on the pharmacokinetics of (-)-carbovir in rats.
Pharm. Res. 13(9) , 1417-8, (1996)
|