Azelaic acid
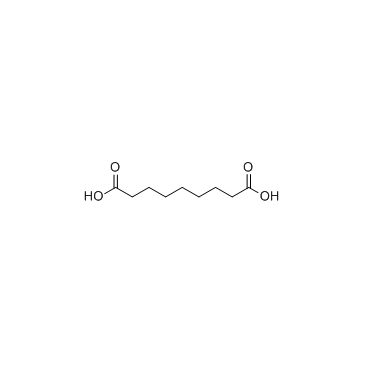
Azelaic acid structure
|
Common Name | Azelaic acid | ||
|---|---|---|---|---|
| CAS Number | 123-99-9 | Molecular Weight | 188.221 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 286 ºC (100 mmHg) | |
| Molecular Formula | C9H16O4 | Melting Point | 98-103 ºC | |
| MSDS | Chinese USA | Flash Point | 215 ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI types (hepatotoxic side effects) seen in the clinic can be tra... |
|
|
Physiology and pathophysiology of organic acids in cerebrospinal fluid.
J. Inherit. Metab. Dis. 16(4) , 648-69, (1993) Concentrations of organic acids in cerebrospinal fluid (CSF) appear to be directly dependent upon their rate of production in the brain. There is evidence that the net release of short-chain monocarboxylic acids from the brain is a major route for removing th... |
|
|
Age-related reference values for urinary organic acids in a healthy Turkish pediatric population.
Clin. Chem. 40(6) , 862-6, (1994) Organic acid concentrations were quantified by gas chromatography and the individual acids identified by mass spectrometry in urine specimens from a healthy Turkish pediatric population of ages 2 days to 16 years, subdivided into five age groups. We quantifie... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain cancer. However, the complete repertoire of signaling pathways ... |
|
|
Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression.
Nature 457(7231) , 910-4, (2009) Multiple, complex molecular events characterize cancer development and progression. Deciphering the molecular networks that distinguish organ-confined disease from metastatic disease may lead to the identification of critical biomarkers for cancer invasion an... |
|
|
Quantitative analysis for organic acids in biological samples: batch isolation followed by gas chromatographic-mass spectrometric analysis.
Clin. Chem. 35(4) , 587-95, (1989) This new method for qualitative and quantitative determination of organic acids, aldehydes, and ketones in biological samples is effective for use with urine, plasma, and amniotic fluid, and it requires no deproteinization. Isolation by batch-wise liquid part... |
|
|
Detection of autosomal dominant polycystic kidney disease by NMR spectroscopic fingerprinting of urine.
Kidney Int. 79(11) , 1244-53, (2011) Autosomal dominant polycystic kidney disease (ADPKD) is a frequent cause of kidney failure; however, urinary biomarkers for the disease are lacking. In a step towards identifying such markers, we used multidimensional-multinuclear nuclear magnetic resonance (... |
|
|
Feasibility of detecting prostate cancer by ultraperformance liquid chromatography-mass spectrometry serum metabolomics.
J. Proteome Res. 13(7) , 3444-54, (2014) Prostate cancer (PCa) is the second leading cause of cancer-related mortality in men. The prevalent diagnosis method is based on the serum prostate-specific antigen (PSA) screening test, which suffers from low specificity, overdiagnosis, and overtreatment. In... |
|
|
Improving the odorant sensitivity of olfactory receptor-expressing yeast with accessory proteins.
Anal. Biochem. 471 , 1-8, (2015) Olfaction depends on the selectivity and sensitivity of olfactory receptors. Previous attempts at constructing a mammalian olfactory receptor-based artificial odorant sensing system in the budding yeast Saccharomyces cerevisiae suffered from low sensitivity a... |
|
|
Mussel-inspired bolaamphiphile sticky self-assemblies for the preparation of magnetic nanoparticles.
Colloids Surf. B Biointerfaces 127 , 89-95, (2015) Adopting the strong metal binding moiety of a mussel protein, a novel bolaamphiphile molecule was prepared and applied to the fabrication of magnetic core-shell nanoparticles. The novel bolaamphiphile molecule with 3,4-dihydroxyphenylalanine (DOPA) end groups... |