Furan-3-carboxylic acid
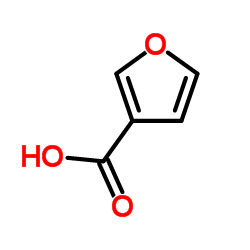
Furan-3-carboxylic acid structure
|
Common Name | Furan-3-carboxylic acid | ||
|---|---|---|---|---|
| CAS Number | 488-93-7 | Molecular Weight | 112.084 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 229.6±13.0 °C at 760 mmHg | |
| Molecular Formula | C5H4O3 | Melting Point | 120-122 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 92.7±19.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect.
Nat. Med. 9(3) , 352-5, (2003) Nicotinic acid (niacin), a vitamin of the B complex, has been used for almost 50 years as a lipid-lowering drug. The pharmacological effect of nicotinic acid requires doses that are much higher than those provided by a normal diet. Its primary action is to de... |
|
|
The hypolipidemic activity of furoic Acid and furylacrylic Acid derivatives in rodents.
Pharm. Res. 2(5) , 233-8, (1985) 2-Furoic acid, 3-furoic acid, 3,4-furan dicarboxylic acid and furyl-acrylic acid were evaluated for hypolipidemic activity in mice and rats. 2-Furoic acid was the most potent agent of the four tested, lowering serum cholesterol levels 41 % and serum triglycer... |
|
|
Basic profiles of organic acids in urine.
J. Chromatogr. A. 525(1) , 1-14, (1990) Altogether 143 of the organic acids regularly occurring in urine of healthy individuals are identified as methyl esters by gas chromatography-mass spectrometry with respect to their complete chemical structures. They are classified as dicarboxylic acids, oxoc... |
|
|
High-performance liquid chromatographic determination of methyl anthranilate, hydroxymethylfurfural and related compounds in honey.
J. Chromatogr. A. 917(1-2) , 95-103, (2001) A high-performance liquid chromatographic method for determining 5-hydroxymethyl-2-furaldehyde (hydroxymethylfurfural), 2-furaldehyde (furfural), furan-2-carboxylic acid (2-furoic acid), furan-3-carboxylic acid (3-furoic acid), furan-3-carboxaldehyde (3-fural... |
|
|
Concurrent formation of furan-2, 5-and furan-2, 4-dicarboxylic acid: unexpected aspects of the Henkel reaction. Thiyagarajan S, et al.
RSC Advances 3(36) , 15678-86, (2013)
|