5-Bromo-2-furaldehyde
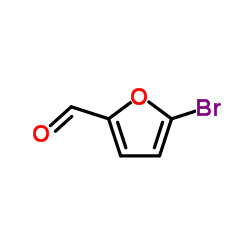
5-Bromo-2-furaldehyde structure
|
Common Name | 5-Bromo-2-furaldehyde | ||
|---|---|---|---|---|
| CAS Number | 1899-24-7 | Molecular Weight | 174.980 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 222.9±20.0 °C at 760 mmHg | |
| Molecular Formula | C5H3BrO2 | Melting Point | 82-85 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 88.6±21.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
5-Substituted-2-furaldehydes: a synthetic protocol utilizing an organozinc route.
J. Org. Chem. 78(5) , 1984-93, (2013) Facile synthetic routes for the preparation of a wide range of 5-substituted 2-furaldehydes have been revealed. They were accomplished through either Pd-catalyzed cross-coupling reaction of various aryl- and heteroarylzinc halides with 5-bromo-2-furaldehyde o... |
|
|
From furans to anilines: toward one-pot two-step amination/diels-alder sequences.
J. Org. Chem. 73(6) , 2191-7, (2008) Selective metal-free amination and Diels-Alder reactions are described in the furan series, leading to polysubstituted anilines or to stable oxabicyclic adducts in high yield. Interestingly, anilines are conveniently prepared through a novel one-pot, two-step... |
|
|
Efficient coupling of heteroaryl bromides with arylboronic acids in the presence of a palladium-tetraphosphine catalyst. Feuerstein M, et al.
Tetrahedron Lett. 42(23) , 5659-62, (2001)
|
|
|
Efficient and Simple Synthesis of 6-Aryl-1,4-dimethyl-9H-carbazoles. Caruso A, et al.
Molecules 13(6) , 1312-1320, (2008)
|
|
|
A synthesis strategy yielding skeletally diverse small molecules combinatorially. Burke MD, et al.
J. Am. Chem. Soc. 126(43) , 14095-14104, (2004)
|
|
|
Furan derivatives. LXXXV11. The synthesis and ultraviolet spectra of 5-(4-X-phenyIsulfonyI)-2-furaldehydes and 2-cyano-3-[5-(4-X-phenyl-sulfonyl)-2-furyl] acrylonitriles. Kada R and Kovác J.
Chemical Papers (Slovak Acad. Sci.) 30(4) , 502-507, (1976)
|
|
|
Photochemical coupling between halogenoheterocyclic and heterocyclic derivatives. D'Agostini A and D'Auria M.
J. Chem. Soc. Perkin Trans. I 9 , 1245-1249, (1994)
|
|
|
Aminomethylations via cross-coupling of potassium organotrifluoroborates with aryl bromides. Molander GA and Sandrock DL.
Org. Lett. 9(8) , 1597-1600, (2007)
|