Ac-D-Phe-OH
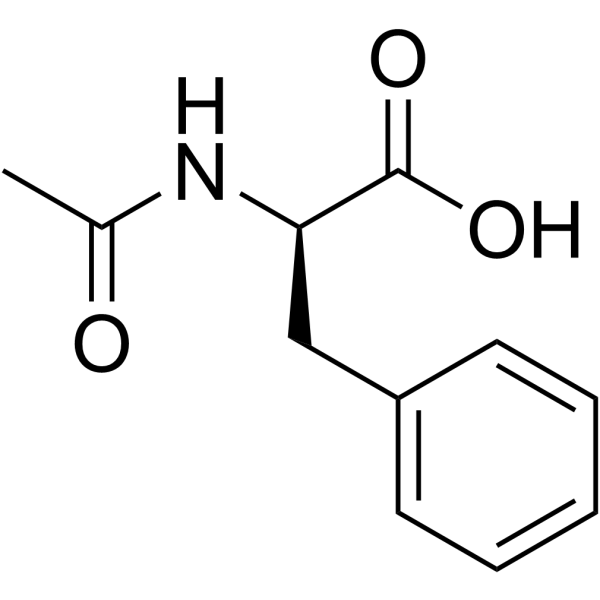
Ac-D-Phe-OH structure
|
Common Name | Ac-D-Phe-OH | ||
|---|---|---|---|---|
| CAS Number | 10172-89-1 | Molecular Weight | 207.226 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 453.9±38.0 °C at 760 mmHg | |
| Molecular Formula | C11H13NO3 | Melting Point | 167ºC | |
| MSDS | Chinese USA | Flash Point | 228.3±26.8 °C | |
|
Peptide synthesis catalyzed by an antibody containing a binding site for variable amino acids.
Science 265 , 234, (1994) Monoclonal antibodies, induced with a phosphonate diester hapten, catalyzed the coupling of p-nitrophenyl esters of N-acetyl valine, leucine, and phenylalanine with tryptophan amide to form the corresponding dipeptides. All possible stereoisomeric combination... |
|
|
Studies on the mechanism for renal elimination of N-acetylphenylalanine: its pathophysiologic significance in phenylketonuria.
J. Lab. Clin. Med. 105(1) , 132-8, (1985) To elucidate the mechanism and biologic significance of urinary occurrence of N-acetylphenylalanine in phenylketonuria, the metabolic fate of N-acetylphenylalanine was studied in rats. In vivo and in vitro analysis revealed that N-acetyl-14C(ul)-phenylalanine... |
|
|
Molecular dynamics (MD) simulations for the prediction of chiral discrimination of N-acetylphenylalanine enantiomers by cyclomaltoheptaose (beta-cyclodextrin, beta-CD) based on the MM-PBSA (molecular mechanics-Poisson-Boltzmann surface area) approach.
Carbohydr. Res. 339(11) , 1961-6, (2004) Molecular dynamics (MD) simulations were performed for the prediction of chiral discrimination of N-acetylphenylalanine enantiomers by cyclomaltoheptaose (beta-cyclodextrin, beta-CD). Binding free energies and various conformational properties were obtained u... |
|
|
13C NMR spectroscopic analysis on the chiral discrimination of N-acetylphenylalanine, catechin and propranolol induced by cyclic-(1-->2)-beta-D-glucans (cyclosophoraoses).
Carbohydr. Res. 337(19) , 1785-9, (2002) Cyclosophoraoses (cyclic-(1-->2)-beta-D-glucans) produced by Rhizobium meliloti were used as a novel chiral NMR solvating agent. 13C NMR spectroscopic analysis as an enantiodiscriminating tool was carried out where NMR signal splittings were observed on the i... |
|
|
Stereoselective formation of bis(alpha-aminoacyl) esters of 5'-AMP suggests a primitive peptide synthesizing system with a preference for L-amino acids.
Biochim. Biophys. Acta 1076(3) , 395-400, (1991) In the biosynthesis of proteins, each amino acid passes from the aminoacyl adenylate to become an amino acid ester and finally a 2' (3') peptidyl ester of the AMP residue at the end of a tRNA. Consequently, the chemistry of protein synthesis is the chemistry ... |
|
|
Signature of the conformational preferences of small peptides: a theoretical investigation.
J. Phys. Chem. A 111(35) , 8650-8, (2007) An extensive computational study of the conformational preferences of N-acetylphenylalaninylamide (NAPA) is reported, including conformational and anharmonic frequency analyses, as well as calculations of excitation energies of the four NAPA conformers lowest... |
|
|
Importance of product inhibition in the kinetics of the acylase hydrolysis reaction by differential stopped flow microcalorimetry.
Anal. Biochem. 308 , 285-293, (2002) The hydrolysis of N-acetyl-L-methionine, N-acetylglycine, N-acetyl-L-phenylalanine, and N-acetyl-L-alanine at 298.35K by porcine kidney acylase I (EC 3.5.1.14) was monitored by the heat released upon mixing of the substrate and enzyme in a differential stoppe... |
|
|
Temperature dependence of the kinetics of the acylase hydrolysis reaction by differential stopped flow microcalorimetry.
Anal. Biochem. 321(1) , 1-7, (2003) The rates of the hydrolysis of N-acetylglycine, N-acetyl-L-methionine, and N-acetyl-L-phenylalanine by porcine acylase I in 0.1M phosphate buffer, which is inhibited by acetate product formation, were monitored calorimetrically at temperatures between 15.2 an... |
|
|
Acid dissociation constant and apparent nucleophilicity of lysine-501 of the alpha-polypeptide of sodium and potassium ion activated adenosinetriphosphatase.
Biochemistry 28(17) , 6894-9, (1989) A combination of competitive labeling with [3H]acetic anhydride [Kaplan, H., Stevenson, K. J., & Hartley, B. S. (1971) Biochem. J. 124, 289-299] and immunoaffinity chromatography is described that permits the assignment of the acid dissociation constant and t... |
|
|
Conformational energies of substrates and inhibitors for carboxypeptidase A: stereoelectronic effect.
J. Biomol. Struct. Dyn. 12(5) , 1033-40, (1995) Because of the complexity involved in binding of a ligand to an enzyme the conformational preference of the bound ligand has not been well understood yet. We have examined the conformational energies of ligands for carboxypeptidase A using ab initio calculati... |