Isophorone
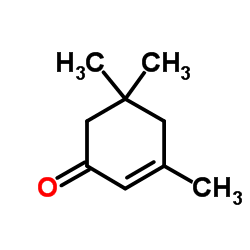
Isophorone structure
|
Common Name | Isophorone | ||
|---|---|---|---|---|
| CAS Number | 78-59-1 | Molecular Weight | 138.207 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 215.2±0.0 °C at 760 mmHg | |
| Molecular Formula | C9H14O | Melting Point | −8 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 84.4±0.0 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
|
Polymer Coatings in 3D-Printed Fluidic Device Channels for Improved Cellular Adherence Prior to Electrical Lysis.
Anal. Chem. 87 , 6335-41, (2015) This paper describes the design and fabrication of a polyjet-based three-dimensional (3D)-printed fluidic device where poly(dimethylsiloxane) (PDMS) or polystyrene (PS) were used to coat the sides of a fluidic channel within the device to promote adhesion of ... |
|
|
Non-targeted multi-component analytical surveillance of plastic food contact materials: Identification of substances not included in EU positive lists and their risk assessment.
Food Addit. Contam. 22(10) , 1012-22, (2005) A procedure used by the Norwegian Food Safety Authority for surveillance of contaminants from plastic food contact materials (polyolefin drinking bottles, water boilers, polyamide cooking utensils and plastic multi-layer materials) is described. It is based o... |
|
|
The synthesis and spectral properties of a stimuli-responsive D-π-A charge transfer dye.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 78(1) , 234-7, (2011) A new donor-π-acceptor (D-π-A) type isophorone dye was synthesized by the condensation reaction between 2-(3,5,5-trimethylcyclohex-2-enylidene)-malononitrile and indole-3-carboxaldehyde. The chemical structure of the dye was characterized by 1H NMR, EA and MS... |
|
|
Determination of isophorone in food samples by solid-phase microextraction coupled with gas chromatography-mass spectrometry.
J. Chromatogr. A. 1155(1) , 100-4, (2007) A simple and sensitive method for the determination of isophorone in food samples was developed by headspace solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS). Isophorone was separated within 10 min by GC-MS using... |
|
|
Toxicology and carcinogenesis studies of isophorone in F344 rats and B6C3F1 mice.
Toxicology 39(2) , 207-19, (1986) Toxicology and carcinogenesis studies of isophorone were conducted by administering 0, 250, or 500 mg/kg body weight per day by gavage in corn oil to groups of 50 F344/N rats and 50 B6C3F1 mice of each sex, 5 days/week, for 103 weeks. Dosed male rats develope... |
|
|
Antifungal activity and biotransformation of diisophorone by Botrytis cinerea.
J. Agric. Food Chem. 53(15) , 6035-9, (2005) Diisophorone (1) was tested against two strains of the necrotrophic plant pathogen Botrytis cinerea. Fungal sensitivity varied according to the strain. B. cinera 2100 was more sensitive than B. cinereaUCA992: its mycelial growth was significantly inhibited at... |
|
|
Model of drug-loaded fluorocarbon-based micelles studied by electron-spin induced (19)f relaxation NMR and molecular dynamics simulation.
Langmuir 24(3) , 692-700, (2008) Rf-IPDU-PEGs belong to a class of fluoroalkyl-ended poly(ethylene glycol) polymers (Rf-PEGs), where the IPDU (isophorone diurethane) functions as a linker to connect each end of the PEG chain to a fluoroalkyl group. The Rf-IPDU-PEGs form hydrogels in water wi... |
|
|
Rapid toxicity testing based on mitochondrial respiratory activity.
Bull. Environ. Contam. Toxicol. 44(5) , 675-80, (1990)
|
|
|
Solid-state linear-dichroic IR-spectroscopy of isophorone derivatives with potential non-linear optical application.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 65(5) , 1035-40, (2006) Possibilities of linear-dichroic infrared (IR-LD) spectroscopy based on oriented solid samples as suspension in nematic liquid crystal have been applied for detailed experimental IR-band assignment and structural information of 2-[5,5-dimethyl-3-(2-phenyl-vin... |
|
|
Biotransformation of racemic diisophorone by Cephalosporium aphidicola and Neurospora crassa.
Biotechnol. Lett. 27(14) , 1007-10, (2005) Racemic diisophorone (500 mg) was converted by Cephalosporium aphidicola and Neurospora crassa over 10 days at 25 degrees C to 8beta-hydroxydiisophorone in yields of 10% (52 mg) and 20% (103 mg), respectively. The structure was established by IR, specific rot... |