4-Methoxy-TEMPO
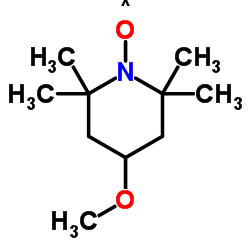
4-Methoxy-TEMPO structure
|
Common Name | 4-Methoxy-TEMPO | ||
|---|---|---|---|---|
| CAS Number | 95407-69-5 | Molecular Weight | 186.271 | |
| Density | N/A | Boiling Point | 242.1ºC at 760mmHg | |
| Molecular Formula | C10H20NO2 | Melting Point | 40.5-44ºC | |
| MSDS | Chinese USA | Flash Point | 100.2ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
|
Oxidation of 4-substituted TEMPO derivatives reveals modifications at the 1- and 4-positions.
Org. Biomol. Chem. 9(13) , 4936-47, (2011) Potenital pathways for the deactivation of hindered amine light stabilisers (HALS) have been investigated by observing reactions of model compounds--based on 4-substituted derivatives of 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO)--with hydroxyl radicals. In... |
|
|
Efficient aerobic oxidative synthesis of 2-substituted benzoxazoles, benzothiazoles, and benzimidazoles catalyzed by 4-methoxy-TEMPO.
Angew. Chem. Int. Ed. Engl. 47(48) , 9330-3, (2008)
|
|
|
Studies on bio-antioxidants--micellar effects on the reduction of nitroxides by vitamin C.
Sci. China,. Ser. B, Chem. Life Sci. Earth Sci. 32(8) , 937-47, (1989) The kinetics of reduction of nitroxides including 4-hydroxy-TEMPO, 4-methoxy-TEMPO and 4-hexanoyloxy-TEMPO, which are of different lipophilicities, by vitamin C in cationic, non-ionic and anionic micelles, i.e. CTAB, Triton X-100 and SDS, respectively, have b... |
|
|
Metexyl (4-methoxy-2,2,6,6-tetramethylpiperidine-1-oxyl) as an oxygen radicals scavenger and apoptosis inducer in vivo.
Anticancer Res. 19(6B) , 5259-64, (1999) A stable nitroxide radical named Metexyl (4-methoxy-2,2,6,6-tetramethylpiperidine-1-oxyl) was synthesized and its antioxidant and antitumor properties were investigated and compared with these of another nitroxide derivatives previously designed in our labora... |
|
|
Efficient pyrimidine N-1-alkylation via activation of electron rich olefins with oxoammonium salts: synthesis of methoxy TEMPO substituted pyrimidine nucleoside analogs.
Nucleosides Nucleotides Nucleic Acids 23(11) , 1723-38, (2004) Our work outlines the use of oxoammonium salts in a formal 1,2 addition process to olefins giving nucleoside analogs as products. Specifically, oxoammonium salts can be added to a solution of olefin and silylated heterocycle to give Methoxy TEMPO substituted ... |