nessler's reagent
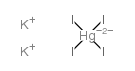
nessler's reagent structure
|
Common Name | nessler's reagent | ||
|---|---|---|---|---|
| CAS Number | 7783-33-7 | Molecular Weight | 786.40400 | |
| Density | 1.16 g/mL at 20 °C | Boiling Point | N/A | |
| Molecular Formula | HgI4K2 | Melting Point | 120-127 °C(lit.) | |
| MSDS | N/A | Flash Point | >230 °F | |
| Symbol |




GHS05, GHS06, GHS08, GHS09 |
Signal Word | Danger | |
|
Purification of histone core octamers and 2.15 A X-ray analysis of crystals in KCl/phosphate.
Acta Crystallogr. D Biol. Crystallogr. 55(Pt 5) , 1048-51, (1999) Intact histone octamers, produced by a new method quickly and in bulk, were crystallized in KCl/phosphate, and the X-ray data were analysed to 2.15 A, confirming a P65 space group. This environment preserves the high-resolution structure of the octamers and w... |
|
|
Indirect atomic absorption determination of atropine, diphenhydramine, tolazoline, and levamisole based on formation of ion-associates with potassium tetraiodometrcurate.
J. Pharm. Biomed. Anal. 25(1) , 3-7, (2001) Ion-associate complexes of atropine sulphate (I), diphenhydramine HCl (II), tolazoline HCl (III) and levamisole HCl (IV) with potassium tetraiodomercurate were precipitated and their solubilities were studied as a function of pH, ionic strength and temperatur... |
|
|
A simple diffuse reflectance Fourier transform infrared spectroscopic determination of sulfur dioxide in ambient air using potassium tetrachloromercurate as the absorbing reagent.
J. AOAC Int. 90(4) , 1180-90, (2007) This paper presents development of a simple, rapid, and precise analytical method for determination of sulfur dioxide in ambient air by a gas to solid-phase conversion method. Sulfur dioxide is determined in the form of sulfite (SO3(2-)) because the absorbing... |
|
|
E.A. Burns, C.A. Streuli, P.R. Averell, eds.
The Analytical Chemistry of Nitrogen and its compounds New York , 51, (1970)
|