137281-23-3
| 中文名 | 培美曲塞 |
|---|---|
| 英文名 | pemetrexed |
| 中文别名 |
N-(4-[2-(2-氨基-4,7-二氢-4-氧-1H-吡咯[2,3-d]嘧啶-5-基)乙基]苯甲酰)-L-谷氨酸
培美曲赛酸 N-[4-[2-(2-胺基-4,7-二氢-4-氧-1H吡咯[2,3-D]嘧啶-5基)乙基]苯甲酰]-L-谷氨酸 培美曲塞二钠盐 培美曲塞酸 |
| 英文别名 |
n-[4-[2-(2-amino-4,7-dihydro-4-oxo-3h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]l-glutamic acid
Pemetrexed N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic Acid Alimta (2S)-2-[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioic acid LYA MFCD00902635 N-{4-[2-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L-glutamic acid Pemetrexed acid N-{4-[2-(2-Amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L-glutamic acid |
| 描述 | Pemetrexed 是一种叶酸拮抗剂 (antifolate)。抑制胸苷酸合成酶 (TS),二氢叶酸还原酶 (DHFR) 和甘氨酰胺核苷酸甲酰转移酶 (GARFT),Ki 分别为 1.3 nM,7.2 nM 和 65 nM。 |
|---|---|
| 相关类别 | |
| 靶点 |
Ki: 1.3 nM (TS), 7.2 nM (DHFR), 65 nM (GARFT)[1] |
| 体外研究 | 培美曲塞(LY231514)二钠是一种新型经典抗叶酸剂,其抗肿瘤活性可能是由于其多聚谷氨酸化代谢物同时和多重抑制几种关键的叶酸需要酶。培美曲塞(LY231514)是已知用于FPGS的最佳底物之一(Km =1.6μM和Vmax/Km = 621)。多糖化和LY231514的多谷氨酸化代谢物可能在确定该新药的选择性和抗肿瘤活性方面发挥重要作用。尽管LY23154仅适度抑制TS(Ki = 340nM,重组小鼠),但LY23154的戊谷氨酸的效力是100倍(Ki = 3.4nM),使得LY231514成为最有效的基于叶酸的TS抑制剂之一[1]。 |
| 体内研究 | 用PC61加培美曲塞治疗的小鼠组在统计学上显示出比其他组更长的存活期。在生存分析中,与单用PC61,大鼠IgG加培美曲塞治疗或未治疗的患者相比,用PC61加培美曲塞治疗的小鼠组观察到明显更好的生存[2]。 |
| 激酶实验 | 通过在A298监测由10-甲酰基 - [6R,S] -5,6,7,8-四氢叶酸的[6S] -5,6,7,8-四氢叶酸的形成,在室温下进行AICARFT抑制试验。所有溶液在使用前用N 2气体吹扫。反应溶液含有33mM Tris-Cl,pH 7.4,25mM KCl,5mM 2-巯基乙醇,0.05mM AICA核糖核苷酸和16nM(2毫单位/ mL)AICARFT。使用10-甲酰基 - [6R,S] -5,6,7,8-四氢叶酸盐浓度0.037,0.074和0.145mM(分别为其Km值的0.61,1.23和2.45倍)。测试LY231514作为0.08-0.8mM(四种浓度)的抑制剂。当LY231514的三 - 和五谷氨酸盐用作抑制剂时,浓度为0.0005-0.009mM(八种浓度)。通过添加酶引发酶测定。使用ENZFITTER程序分析数据以进行竞争性抑制。 |
| 细胞实验 | 产生剂量 - 反应曲线以确定50%生长抑制所需的浓度(IC 50)。培美曲塞最初以4mg / mL的浓度溶解于DMSO中,并进一步用细胞培养基稀释至所需浓度。将完全培养基中的CCRF-CEM白血病细胞加入到24孔Cluster平板中,终浓度为4.8×10 4细胞/孔,总体积为2mL。将各种浓度的测试化合物加入到两个孔中,使得DMSO的最终体积为0.5%。将板在37℃,5%CO 2的空气气氛中温育72小时。在孵育结束时,在ZBI Coulter计数器上测定细胞数。对照孔通常在孵育结束时含有4×105至6×10 5个细胞。对于一些研究,在300μMAICA,5μM胸苷,100μM次黄嘌呤或5μM嘧啶加100μM次黄嘌呤的组合存在下测定每种化合物的IC50 [1]。 |
| 动物实验 | 小鼠[2]使用6-8周龄的雌性CBA小鼠和雌性NOD / SCID小鼠(NOD.CB17-Prkdcscid)。从第4-8天(连续5天)腹膜内给予预甲状腺素(100mg / kg)至荷瘤小鼠,以探索当与抗CD25Ab或IgG对照组合时的协同效应。在当前研究中用于培美曲塞的剂量和时间表是基于先前在小鼠中的研究确定的。 |
| 参考文献 |
| 密度 | 1.6±0.1 g/cm3 |
|---|---|
| 分子式 | C20H21N5O6 |
| 分子量 | 427.411 |
| 精确质量 | 427.149170 |
| PSA | 191.26000 |
| LogP | -0.03 |
| 外观性状 | 固体;White to Blue powder to crystal |
| 折射率 | 1.724 |
| 储存条件 | 0-10°C;避免加热 |
| 水溶解性 | 微溶:甲醇 |
| 更多 | 1.性状:结晶。 2.熔点(ºC):>250 |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Pemetrexed Synonyms:(S)-2-(4-(2-(2-Amino-4-oxo-4,7-dihydro-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioic acid Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed.
Section3. Composition/information on ingredients. Ingredient name:Pemetrexed 137281-23-3 CAS number: Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Inhalation: Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Storage:Store in closed vessels, refrigerated. Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified No data Boiling point: Melting point:No data Flash point:No data Density:No data Molecular formula:C20H21N5O6 Molecular weight:427.4 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
| 危害码 (欧洲) | Xi |
|---|---|
| WGK德国 | 3 |
| 海关编码 | 2933990090 |
|
~74% 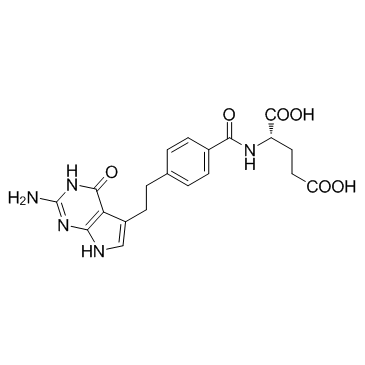
137281-23-3 |
| 文献:Busolli, Jonathan; Diulgheroff, Nicola; Nemethne Racz, Csilla; Pirkes, Moran; Pontiroli, Alessandro; Villa, Marco; Aronhime, Judith Patent: US2008/45711 A1, 2008 ; Location in patent: Page/Page column 11 ; |
| 上游产品 1 | |
|---|---|
| 下游产品 0 | |
| 海关编码 | 2933990090 |
|---|---|
| 中文概述 | 2933990090. 其他仅含氮杂原子的杂环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途, 乌洛托品请注明外观, 6-己内酰胺请注明外观, 签约日期 |
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |



