Pemetrexed disodium
Modify Date: 2025-08-20 19:57:01
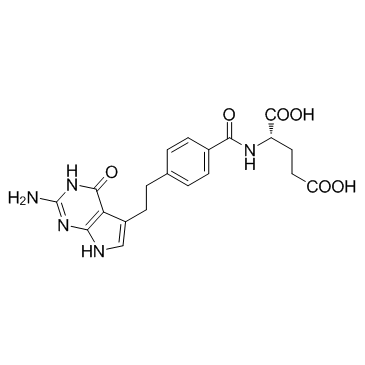
Pemetrexed disodium structure
|
Common Name | Pemetrexed disodium | ||
|---|---|---|---|---|
| CAS Number | 137281-23-3 | Molecular Weight | 427.411 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C20H21N5O6 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Pemetrexed disodiumPemetrexed is a novel antifolate, the Ki values of the pentaglutamate of LY231514 are 1.3, 7.2, and 65 nM for inhibits thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), respectively. |
| Name | pemetrexed |
|---|---|
| Synonym | More Synonyms |
| Description | Pemetrexed is a novel antifolate, the Ki values of the pentaglutamate of LY231514 are 1.3, 7.2, and 65 nM for inhibits thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 1.3 nM (TS), 7.2 nM (DHFR), 65 nM (GARFT)[1] |
| In Vitro | Pemetrexed (LY231514) disodium is a novel classical antifolate, the antitumor activity of which may result from simultaneous and multipie inhibition of several key folate-requiring enzymes via its polyglutamated metabolites. Pemetrexed (LY231514) is one of the best substrates that is known for the enzyme FPGS (Km=1.6 μM and Vmax/Km=621). It is likely that polyglutamation and the polyglutamated metabolites of LY231514 play profound roles in determining both the selectivity and the antitumor activity of this novel agent. Whereas LY23l5l4 only moderately inhibits TS (Ki=340 nM, recombinant mouse), the pentaglutamate of LY23l5l4 is 100-fold more potent (Ki=3.4 nM), making LY231514 one of the most potent folate-based TS inhibitors[1]. |
| In Vivo | The group of mice treated with PC61 plus Pemetrexed demonstrates statistically longer survival than other groups. In a survival analysis, significantly better survival is observed in the group of mice treated with PC61 plus Pemetrexed compare with those treated with PC61 alone, rat IgG plus Pemetrexed, or no treatment[2]. |
| Kinase Assay | AICARFT inhibition assays are carried out at room temperature by monitoring the formation of [6S]-5,6,7,8-tetrahydrofolate from 10-formyl-[6R,S]-5,6,7,8-tetrahydrofolate at A298. All solutions are purged with N2 gas prior to use. The reaction solution contains 33 mM Tris-Cl, pH 7.4, 25 mM KCl, 5 mM 2-Mercaptoethanol, 0.05 mM AICA ribonucleotide, and 16 nM (2 milliunits/mL) of AICARFT. 10-Formyl-[6R,S]-5,6,7,8-tetrahydrofolate concentrations of 0.037, 0.074, and 0.145 mM are used (0.61, 1.23, and 2.45 times its Km value, respectively). LY231514 is tested as an inhibitor at 0.08-0.8 mM (four concentrations). When the tri- and pentaglutamates of LY231514 are used as inhibitors, the concentrations are 0.0005-0.009 mM (eight concentrations). Enzyme assays are initiated by the addition of enzyme. Data is analyzed using the ENZFITTER program for competitive inhibition. |
| Cell Assay | Dose-response curves are generated to determine the concentration required for 50% inhibition of growth (IC50). Pemetrexed is dissolved initially in DMSO at a concentration of 4 mg/mL and further diluted with cell culture medium to the desired concentration. CCRF-CEM leukemia cells in complete medium are added to 24-well Cluster plates at a final concentration of 4.8×104 cells/well in a total volume of 2 mL. Test compounds at various concentrations are added to duplicate wells so that the final volume of DMSO is 0.5%. The plates are incubated for 72 h at 37°C in an atmosphere of 5% CO2 in air. At the end of the incubation, cell numbers are determined on a ZBI Coulter counter. Control wells usually contain 4×105 to 6×105 cells at the end of the incubation. For several studies, IC50s are determined for each compound in the presence of either 300 μM AICA, 5 μM thymidine, 100 μM hypoxanthine, or combination of 5 μM hymidine plus 100 μM hypoxanthine[1]. |
| Animal Admin | Mice[2] Female CBA mice and female NOD/SCID mice (NOD.CB17-Prkdcscid) at 6-8 wk of age are used. Premetrexed (100 mg/kg) is given i.p. from days 4-8 (5 consecutive d) to tumor-bearing mice to explore the synergistic effect when combined with anti-CD25 Ab or IgG control. The dose and schedule used for Pemetrexed in the current study is determined based on previous studies in mice. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C20H21N5O6 |
| Molecular Weight | 427.411 |
| Exact Mass | 427.149170 |
| PSA | 191.26000 |
| LogP | -0.03 |
| Index of Refraction | 1.724 |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Pemetrexed Synonyms:(S)-2-(4-(2-(2-Amino-4-oxo-4,7-dihydro-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioic acid Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed.
Section3. Composition/information on ingredients. Ingredient name:Pemetrexed 137281-23-3 CAS number: Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Inhalation: Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Storage:Store in closed vessels, refrigerated. Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified No data Boiling point: Melting point:No data Flash point:No data Density:No data Molecular formula:C20H21N5O6 Molecular weight:427.4 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Hazard Codes | Xi |
|---|---|
| WGK Germany | 3 |
| HS Code | 2933990090 |
|
~74% 
Pemetrexed disodium CAS#:137281-23-3 |
| Literature: Busolli, Jonathan; Diulgheroff, Nicola; Nemethne Racz, Csilla; Pirkes, Moran; Pontiroli, Alessandro; Villa, Marco; Aronhime, Judith Patent: US2008/45711 A1, 2008 ; Location in patent: Page/Page column 11 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| L-glutamic acid, N-[4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]- |
| n-[4-[2-(2-amino-4,7-dihydro-4-oxo-3h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]l-glutamic acid |
| Pemetrexed |
| L-Glutamic acid, N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]- |
| N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic Acid |
| L-Glutamic acid, N-(4-(2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo(2,3-d)pyrimidin-5-yl)ethyl)benzoyl)- |
| Alimta |
| (2S)-2-[[4-[2-(2-amino-4-oxo-1,7-dihydropyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedioic acid |
| LYA |
| MFCD00902635 |
| N-{4-[2-(2-Amino-4-oxo-4,7-dihydro-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L-glutamic acid |
| Pemetrexed acid |
| N-{4-[2-(2-Amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L-glutamic acid |
![Diethyl 2-(4-(2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl)benzamido)pentanedioate 4-methylbenzenesulfonate structure](https://image.chemsrc.com/caspic/398/165049-28-5.png)

